Single-chain antibody for targeting Reg3A
A single-chain antibody, heavy chain technology, applied in the biological field, can solve problems such as reduced patient survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
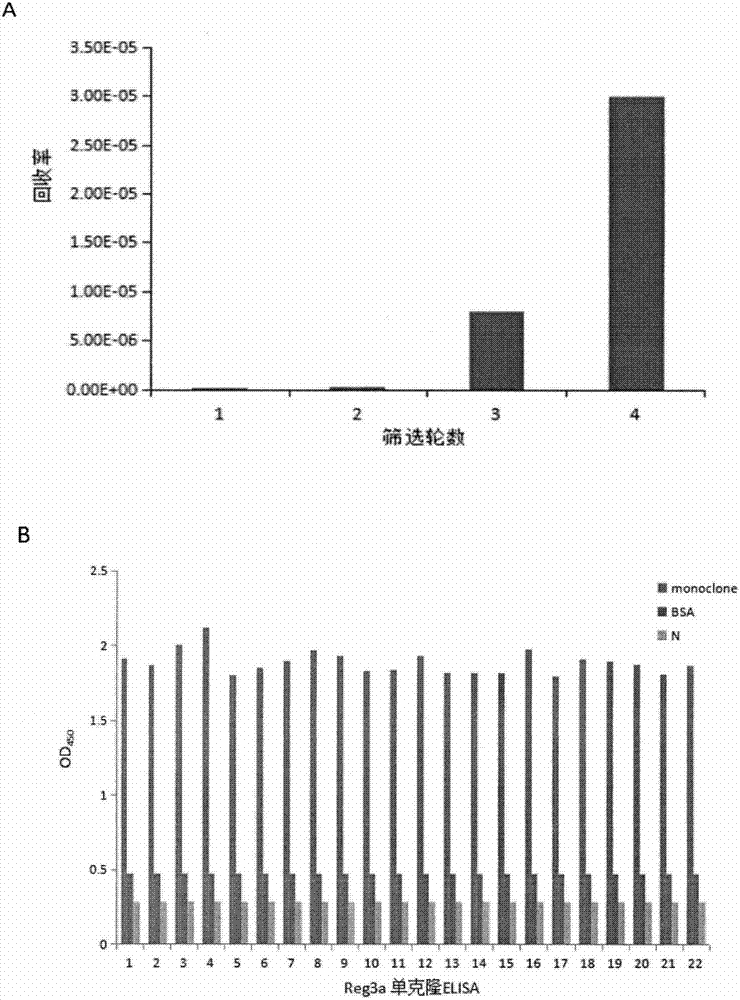
[0029] Example 1 Phage Antibody Library Screening Anti-Reg3A Single Chain Antibody
[0030] 1. Antigen Reg3A
[0031] The Reg3A protein (Q06141-1) used in the present invention is purchased from Beijing Yiqiao Shenzhou Biotechnology Co., Ltd., which contains 175 amino acids and has a purity of >97%.
[0032] 2. Preparation of Escherichia coli host strain TG1 with sex cilia
[0033] TG1 was streaked from the plate in the glycerol cryopreservation tube, inoculated on the M9 medium plate, and cultured upside down at 37°C for 36 hours. Pick a single clonal colony, inoculate it in 5ml 2×YT medium, and cultivate overnight at 37°C on a shaker. The next day, 1 / 100 dilution was inoculated in fresh 2×YT medium, and cultured on a shaker at 37°C to logarithmic phase (OD 600 0.4-0.6), and then infected with phage.
[0034] 3. Preparation of Helper Phage
[0035] Add the helper phage to 200 μL TG1 bacterial culture solution (OD 600 0.5), after 30 minutes in a water bath at 37°C, add it
Embodiment 2
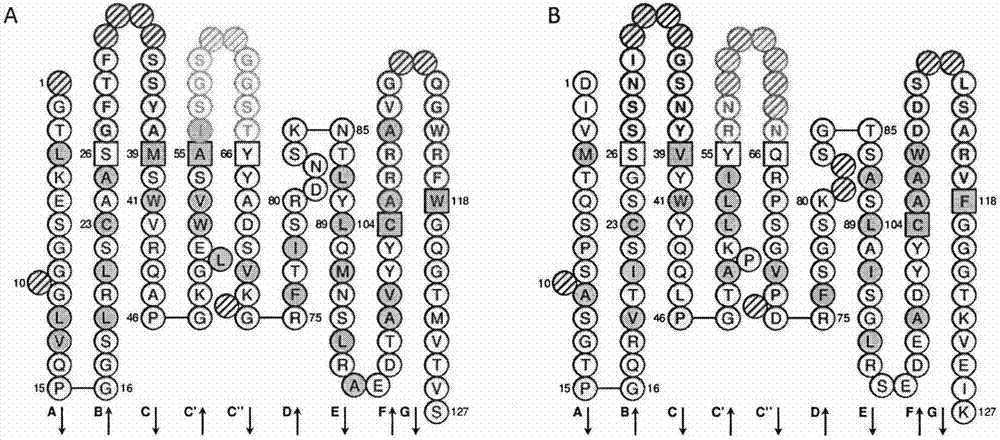
[0055] Example 2 Sequencing and analysis of Reg3A scFv
[0056] Sequencing results showed that five Reg3A scFv gene sequences were successfully obtained, using IMGT / V-QUEST ( http: / / www.imgt.org / ) analyzed the Reg3A scFv gene sequence, and the analysis results showed that: the heavy and light chain variable regions of the Reg3A scFv named A5 and their CDR1-3 region gene sequences are shown in SEQ ID NO.1-8; the Reg3A scFv heavy named C2 1, the gene sequence of the light chain variable region and its CDR1-3 regions are shown in SEQ ID NO.9-16.
Embodiment 3
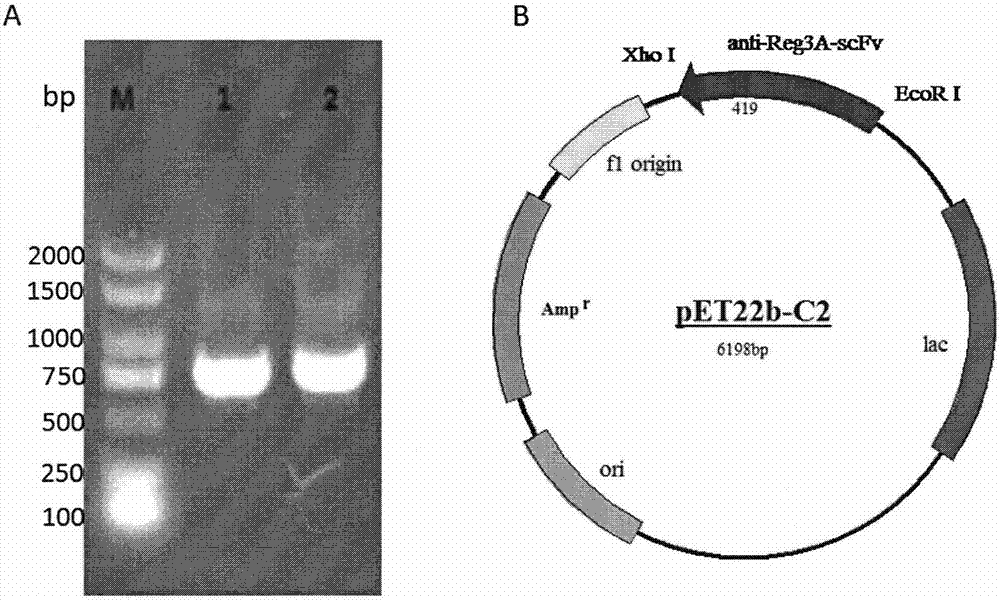
[0057] Preparation and purification of embodiment 3 Reg3A scFv
[0058] PCR amplification of Reg3A scFv gene
[0059] The Reg3A scFv gene was amplified by PCR using the single clone screened from the phage library and correctly sequenced and analyzed as a template. EcoR I and Xho I were selected as upstream and downstream restriction sites respectively, and primers were designed using Primer5.0, wherein the sequences of A5-F, C2-F (upstream primers) and A5-R, C2-R (downstream primers) were:
[0060] A5-F CCGGAATTCCAGGTGGCAGCTGCAGGAGT
[0061] A5-R CCGCTCGAGACGTTTGATATCCACTTTGGTCCC
[0062] C2-F CCGGAATTCTCCAGGTACCTTGAAGGAGTCTGG
[0063] C2-R CCGCTCGAGACGTTTGATCTCCACCTTGGTCC
[0064] The reaction system was 50 μL, and the reaction conditions were 94°C for 4 min, 94°C for 30 s, 57°C for 45 s, 72°C for 1 min, 29 cycles, 72°C for 10 min, and storage at 4°C. Take 5 μL of the final product for identification on 1% agarose gel electrophoresis. Reg3A scFv gene of about 750bp was am
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap