Anti-podoplanin antibody, and pharmaceutical composition containing Anti-podoplanin antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Example
Anti-Podoplanin Antibody)
[0112]The present invention provides an anti-podoplanin antibody for which an epitope is a polypeptide consisting of the amino acid sequence set forth in SEQ ID NO:1 (excluding rat NZ-1 antibody having a heavy chain consisting of the amino acid sequence set forth in SEQ ID NO:2 and a light chain consisting of the amino acid sequence set forth in SEQ ID NO:3, hereinafter, also referred to as “anti-podoplanin antibody of the present invention”).
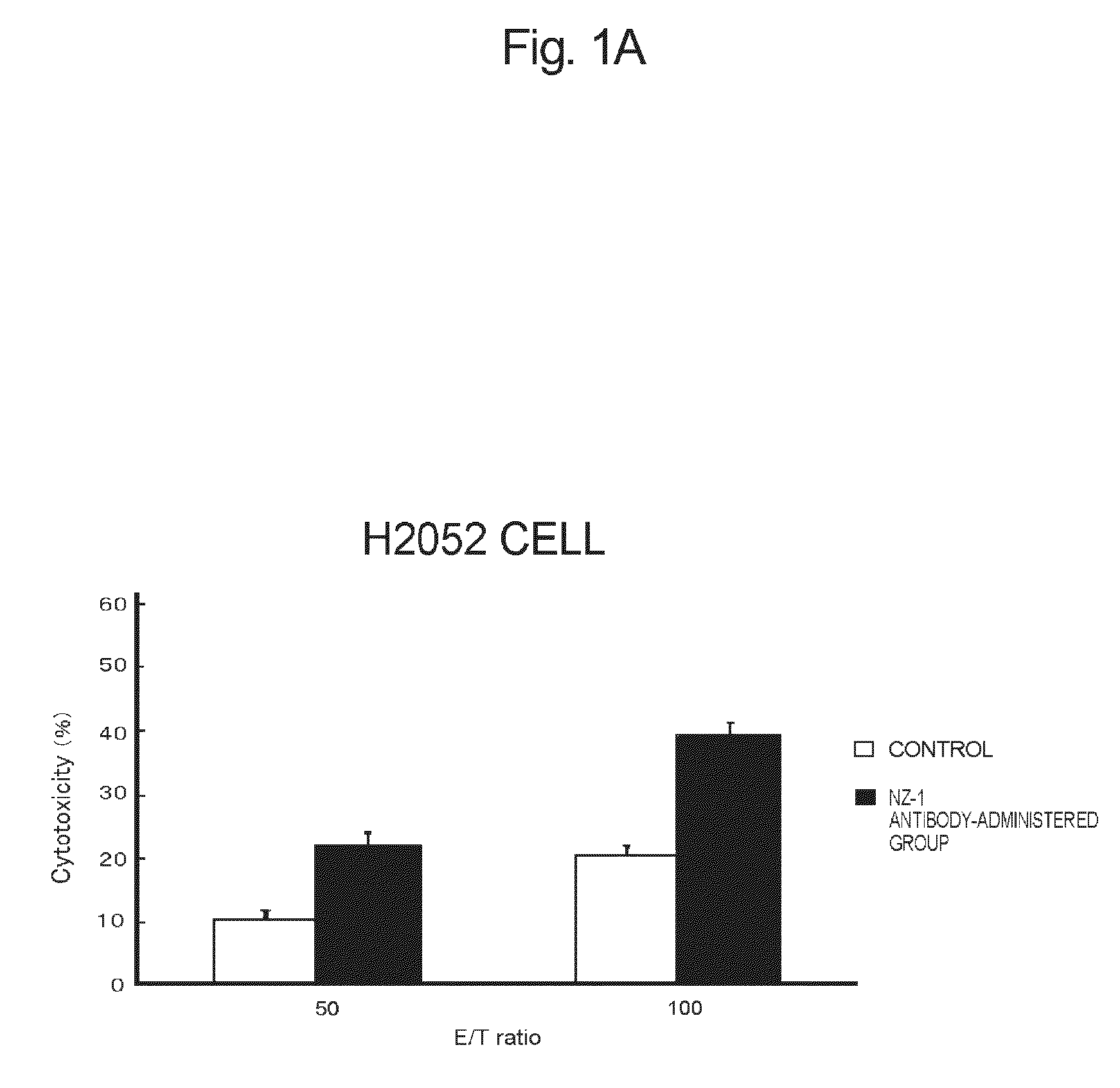
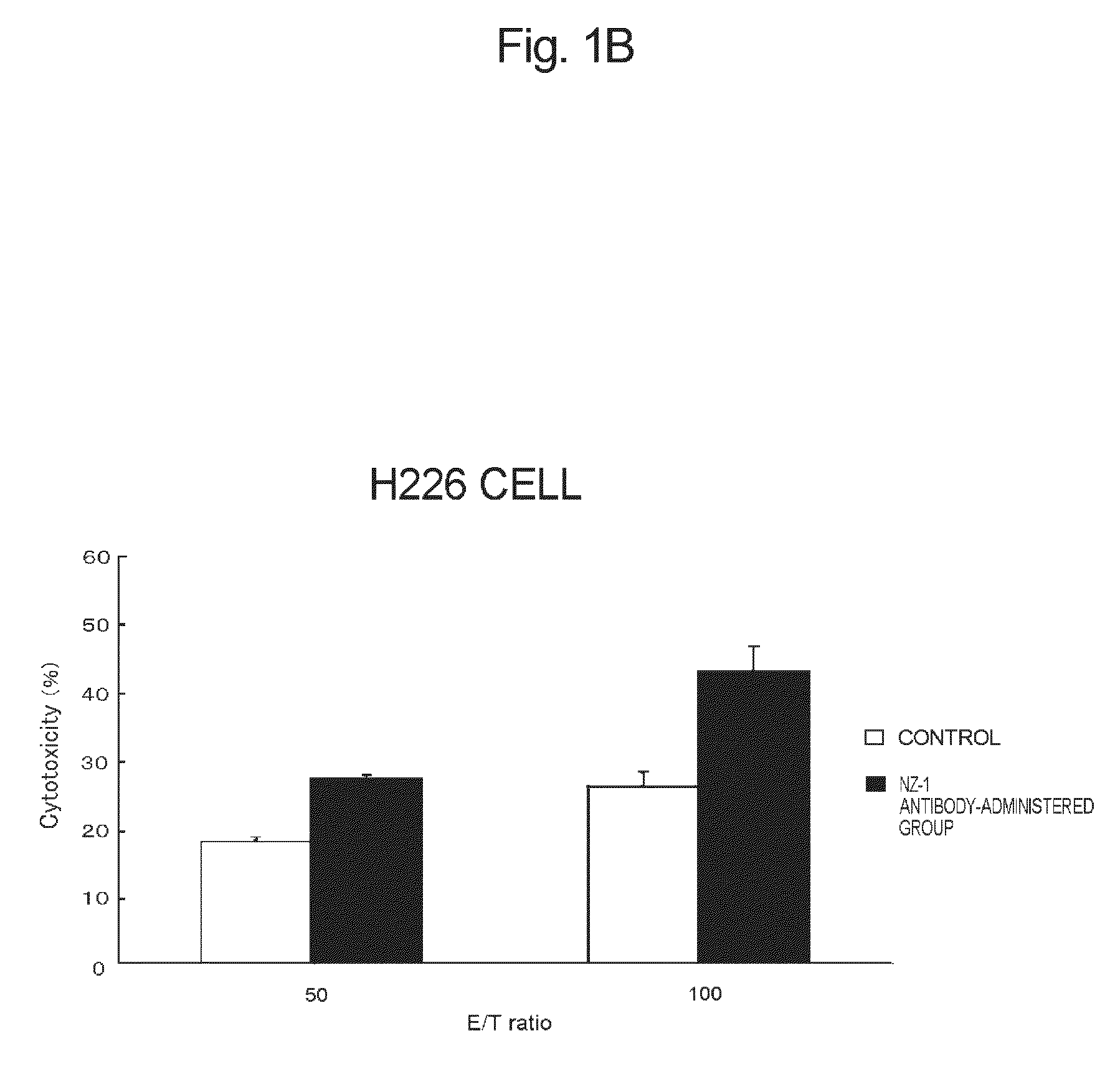
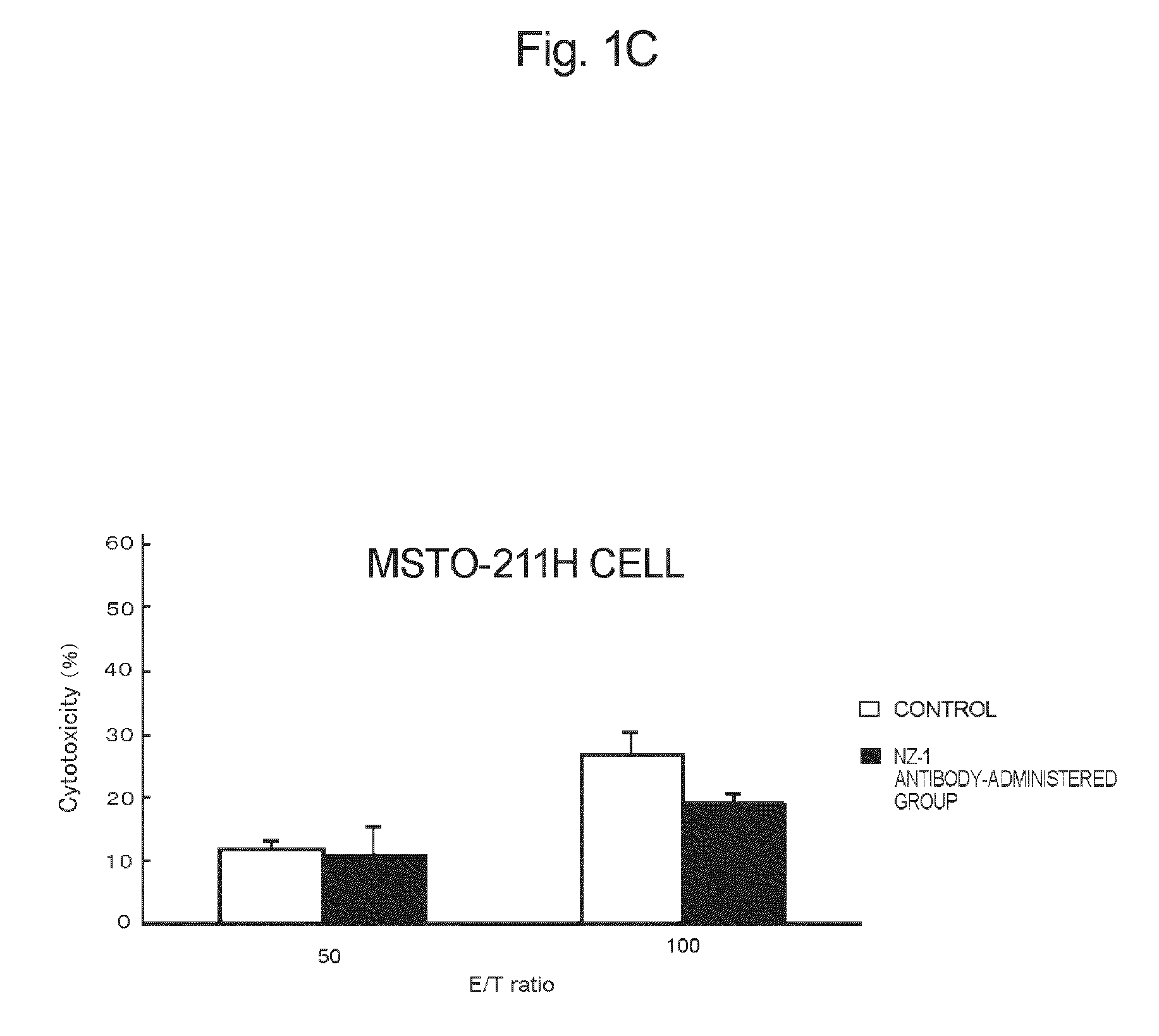
[0113]The anti-podoplanin antibody of the present invention is a novel antibody, inhibits binding of podoplanin to CLEC-2, has high effector activity such ADCC activity or CDC activity, and has a tumor growth-inhibitory potency (which will be described hereinafter).
[0114]There has been known hitherto that rat NZ-1 antibody having a heavy chain consisting of the amino acid sequence set forth in SEQ ID NO:2 and a light chain consisting of the amino acid sequence set forth in SEQ ID NO:3 inhibits binding of podoplanin to CLEC
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Cytotoxicity | aaaaa | aaaaa |
| Inhibition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap