V2O5-LiBO2, V2O5-NiO-LiBO2 GLASSES AND THEIR COMPOSITES OBTAINED BY NITROGEN DOPING AND REDUCED GRAPHITE OXIDE BLENDING AS CATHODE ACTIVE MATERIALS
a cathode active material and composite material technology, applied in the field of glasses, can solve the problems of low storage energy density, lack of stability, and failure to use such oxide materials as cathode materials,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
V2O5—LiBO2 Glass
[0060]The V2O5—LiBO2 glass system is interesting because completely glassy material can be obtained with only 20 wt-% LiBO2, which was not possible with B2O3 as the glass former in earlier trials. A V2O5—LiBO2 glass delivering high specific capacity is shown here for the first time as well as its use as cathode material for Li-ion batteries.
[0061]Although glassy materials with more than 20 wt-% LiBO2 can easily be obtained and are also within the scope of the present invention, glasses with as low as possible LiBO2 content are preferred in view of maximized content of electronically active material.
Synthesis:
[0062]80-20 wt-% V2O5—LiBO2 glass was obtained with a glass synthesis procedure. V2O5 and LiBO2 analytical pure grade powders in corresponding amounts were thoroughly mixed and grinded in an agate mortar, and the mixtures were placed in a Pt crucible. The crucible with the material was put in a muffle furnace at 900° C., and the desired melt was obtained after 1 hou
example 2
V2O5—LiBO2 Glass—Reduced Graphite Oxide (redGO) Composite
[0069]A composite electrode material of V2O5—LiBO2 glass is obtained by the reduction of graphite oxide to graphite and amorphous carbon in a mixture of the glass and graphite oxide. The composite electrode material gives higher practical specific capacity, and the cycling is much improved compared to plain V2O5—LiBO2 glass.
Synthesis:
[0070]20 wt-% graphite oxide and 80 wt-% V2O5—LiBO2 were ball-milled together and the resulting mixture was heated at 200° C. for 8 hours under N2 flow or in air to ensure the reduction of graphite oxide to graphite and amorphous carbon. The carbon content was found to be 10.9 wt-% at the end of the process.
Characterization:
XRD Powder Diffraction and DTA:
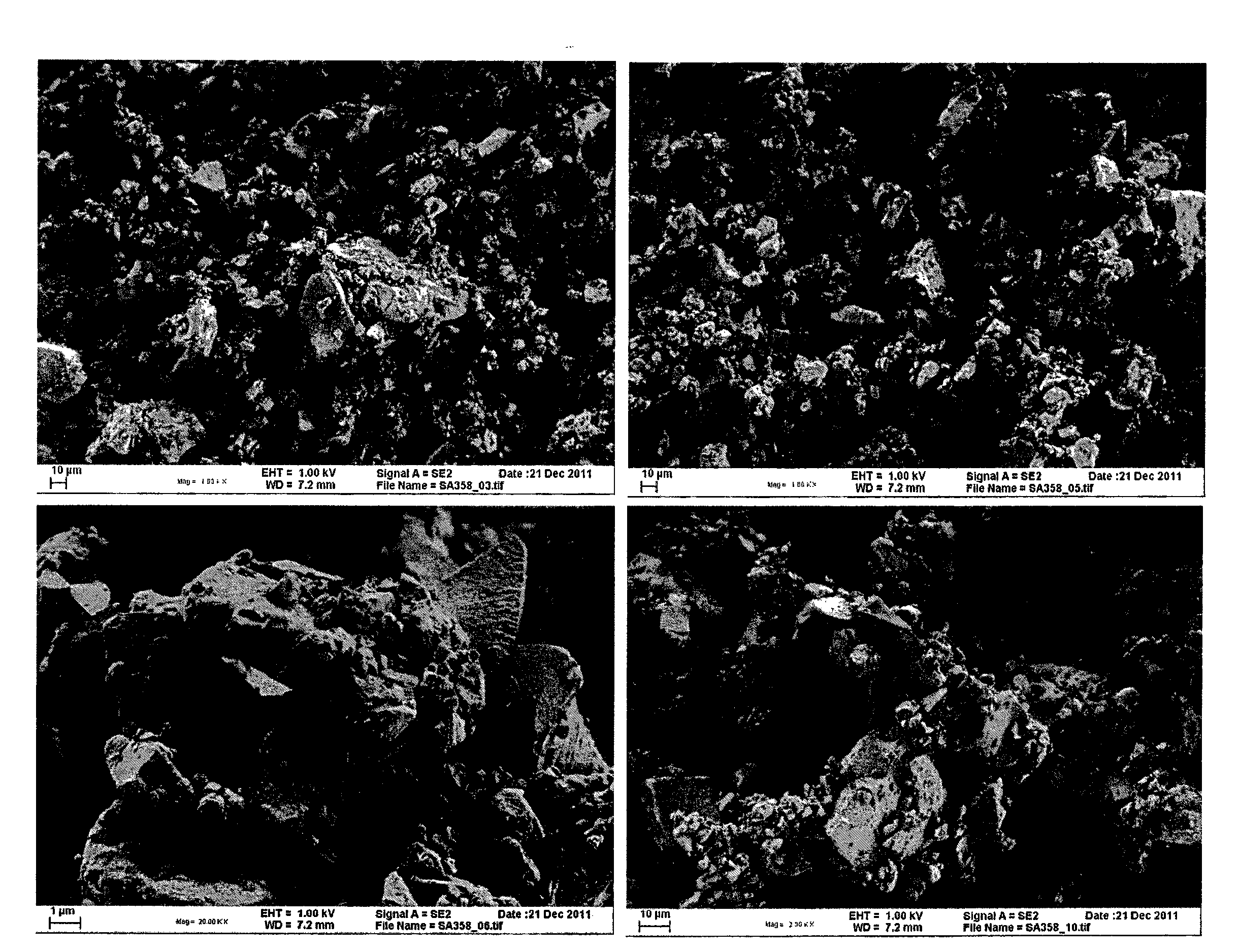
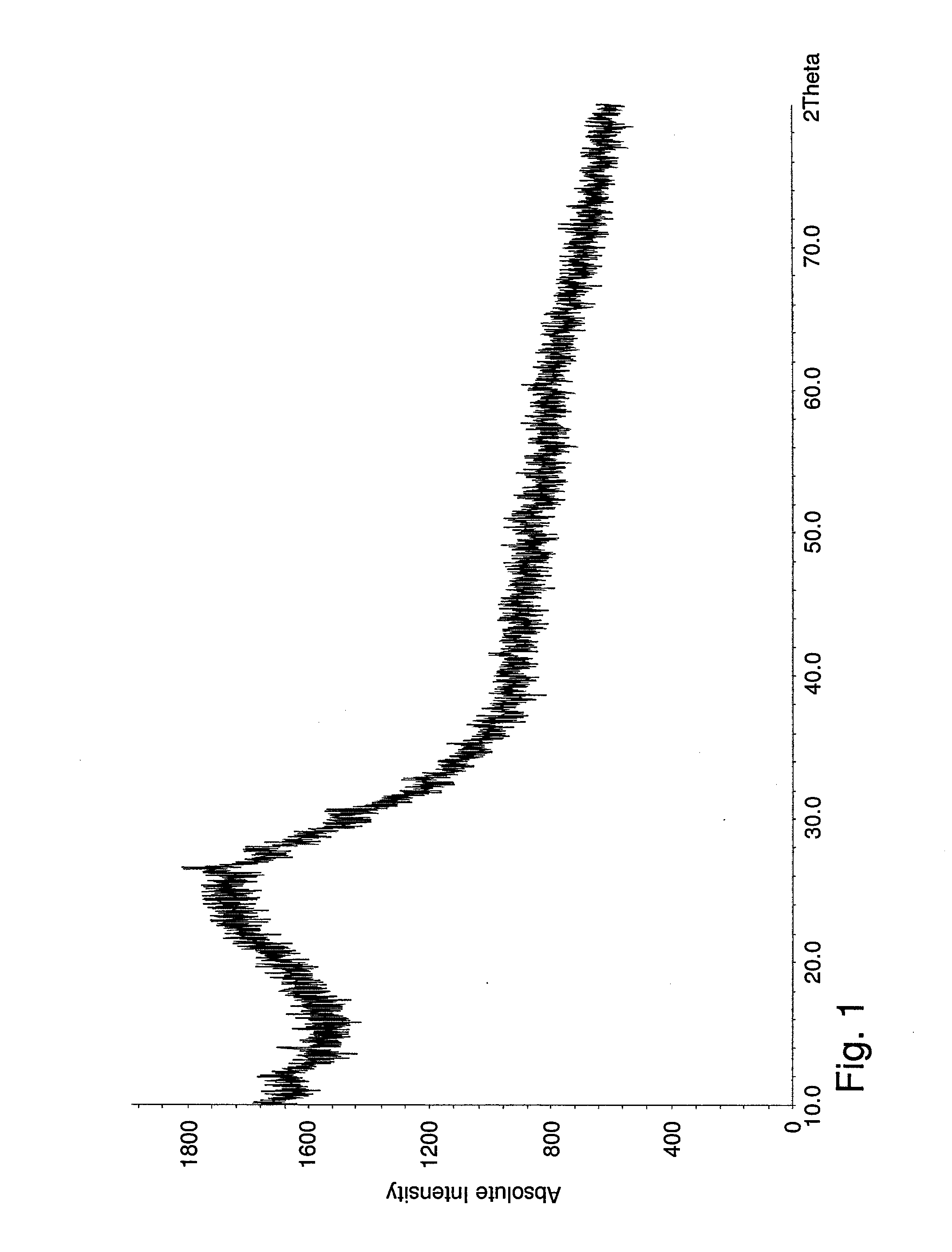
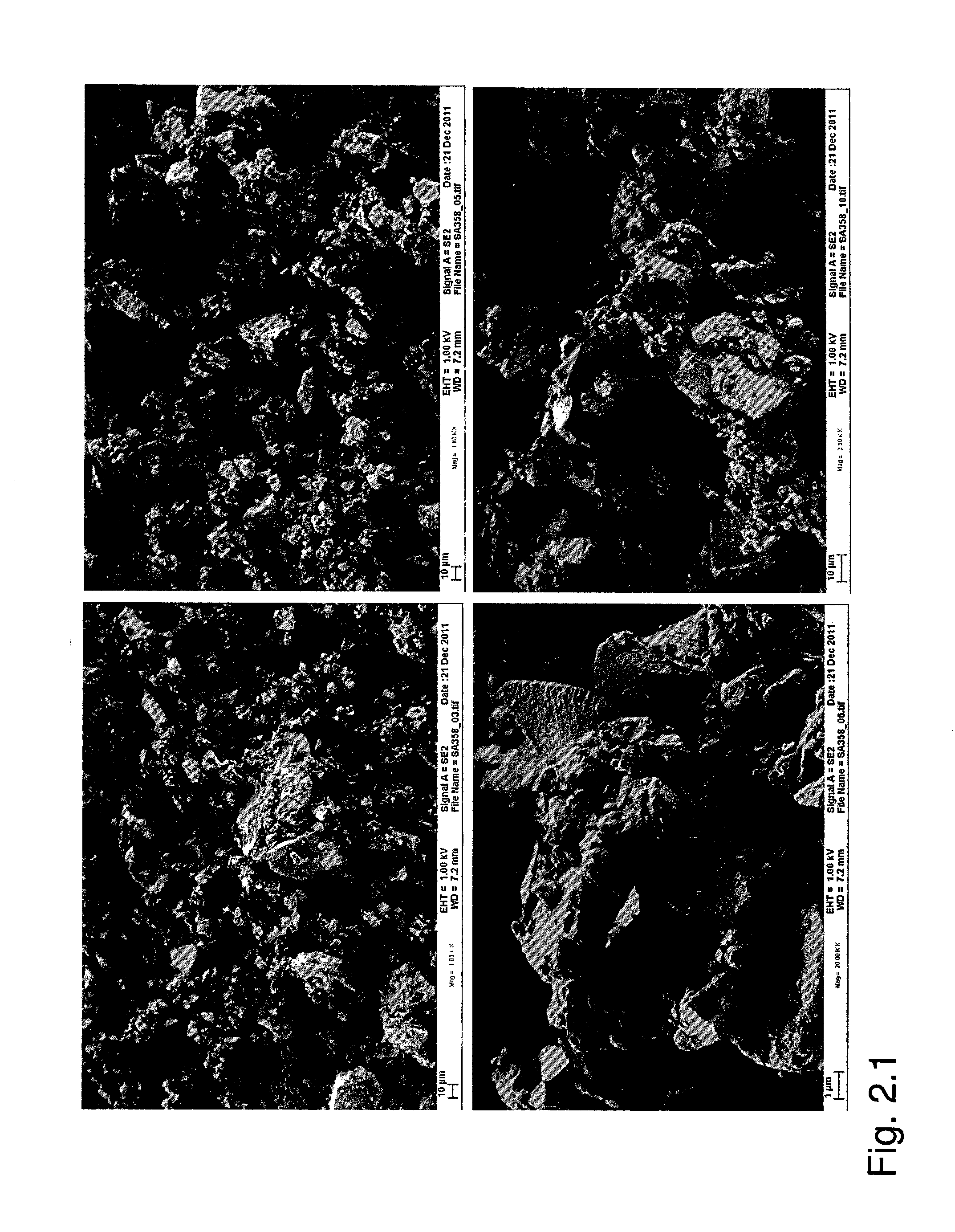
[0071]After ball-milling and heat treatment, a black powder was obtained. No diffraction peak from graphite oxide or graphite was observed. The absence of a graphite peak was attributed to the thin layers formed. The crystallinity of the material was
example 3
V2O5-MnOv—LiBO2 Glass—Reduced Graphite Oxide Composite, in Particular V2O5—NiO—LiBO2 Glass—Reduced Graphite Oxide Composite
[0076]In order to improve the voltage range of the glass system ternary and quaternary glass systems of the inventive type, in particular V2O5 glass systems where LiBO2 is used as the glass former, can be synthesized with M in MuOv being high voltage redox couples, such as Co2+ / Co3+ and Ni2+ / Ni3+. As an alternative or in combination with the high voltage redox couples the structural integrity upon cycling can be improved by incorporating a compound like Na2O and / or Al2O3 as it was previously shown for NMC (nickel-mangan-cobaltoxide) materials.
[0077]V2O5—Na2O—LiBO2, V2O5—Co3O4—LiBO2, V2O5—Co3O4—Al2O3—LiBO2, and V2O5—NiO—LiBO2 glasses were successfully synthesized. Among these systems, the best results so far were obtained with V2O5—NiO—LiBO2. Therefore V2O5—NiO—LiBO2 is further described below.
Synthesis:
[0078]80-5-15 wt-% V2O5—NiO—LiBO2 glass was obtained as d
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
- T is a transition metal selected from V and Mo,
- M is a metal selected from Ni, Co, Na, Al, Mn, Cr, Cu, Fe, Ti and mixtures thereof,
- x, y, u, and v are the stoichiometric coefficients resulting in a neutral compound, i.e. x=2y/(oxidation state of T) and u=2v/(oxidation state of M),
- z, w and t are weight-%, wherein
- z is 70-80,
- w is 0-20
- t is 10-30, and
- the sum of z, w and t is 100 weight-%, in particular V2O5—LiBO2 and V2O5—NiO—LiBO2.
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap