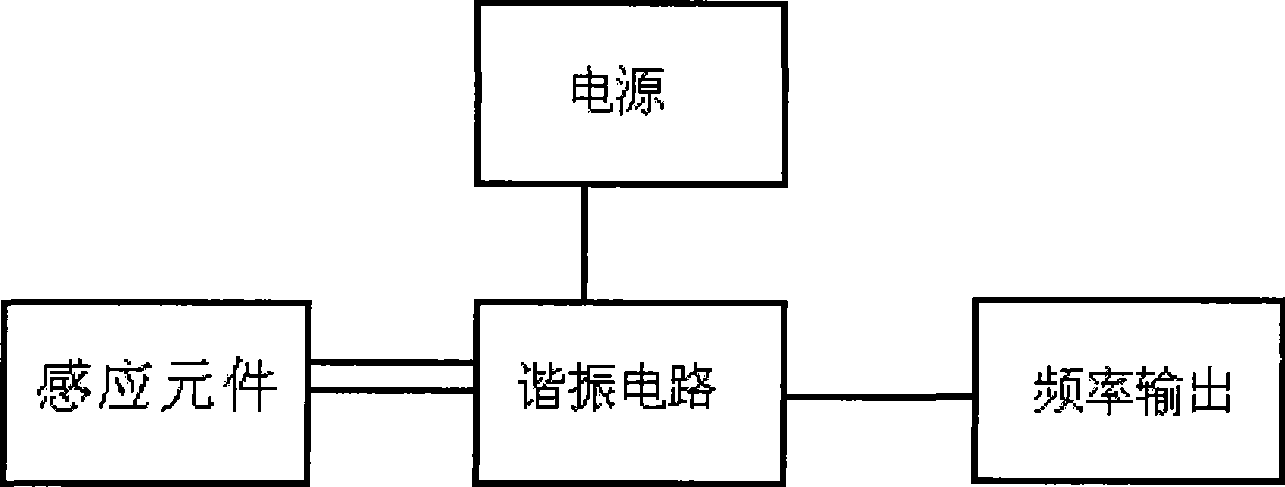
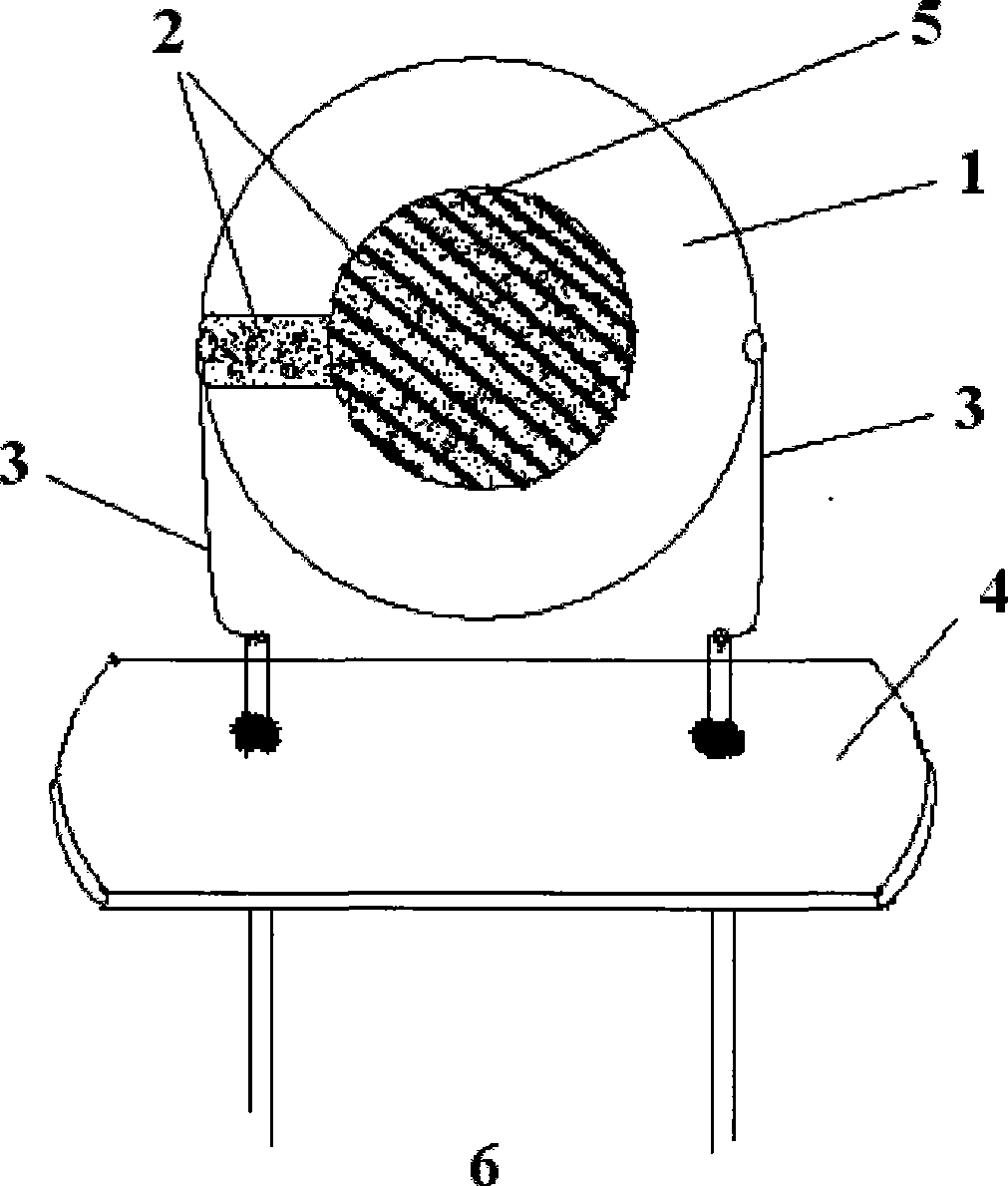
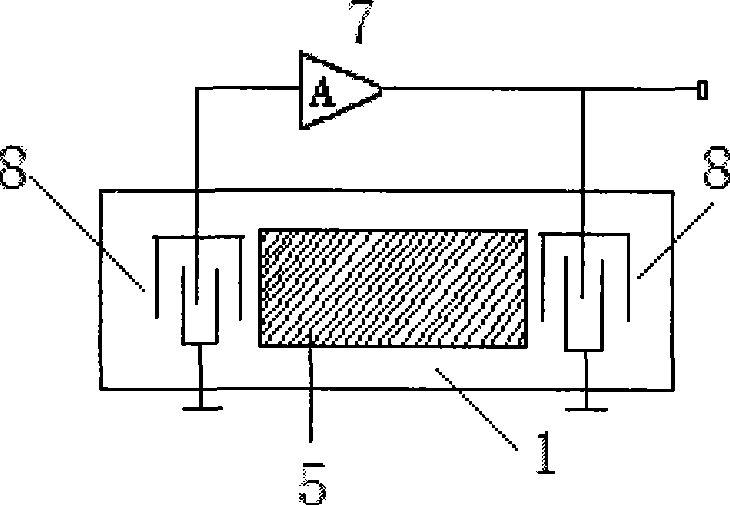
Characterization method for ionic liquid dissolvability
A technology of ionic liquid and solubility, which is applied in the field of characterization of the interaction between ionic liquid and gas at room temperature, can solve the problems of up to 1-3 hours, long measurement period, etc., achieve fast solubility, reliable data, and promote application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1), 10MHz QCM quartz crystal resonator in absolute ethanol, ultrasonic cleaning, drying, QCM measurement fundamental frequency, record the precise frequency value f 0 =9992120Hz, package the chip for use;
[0034] (2), accurately weigh 70mg of ionic liquid C 4 mimCl was dissolved in 7 mL of chloroform to prepare a 10 mg / mL coating solution.
[0035] (3), the cleaned 9992120Hz quartz crystal oscillator is suspended in 10mg / mL ionic liquid C 4 Soak in the chloroform solution of mimCl for 30 sec, and wait for the volatile organic solvent chloroform on the surface of the wafer to volatilize completely.
[0036] (4) Purging the coated quartz crystal oscillator at 60°C with 60mL / min of high-purity nitrogen, the frequency is stable for 1 to 2 hours, and the frequency f is measured on the QCM 1 =9987750Hz, then the frequency reduction value |Δf|=|f 1 -f 0 |=|9987550Hz-9992120Hz|=4550Hz is the decrease in oscillation frequency caused by a slight increase in wafer qualit
Embodiment 2
[0039] Using the ionic liquid C prepared in Example 1 4 mimCl is a QCM oscillator with sensitive membrane material, which detects different concentrations of ethanol gas respectively. The measured gas concentration and the frequency change value of the response are listed in Table 1. The Hi values are also listed in Table 1 accordingly, and the measured H i The average value is 2.02kPa, the relative standard deviation is 3.4%, n=8.
[0040] Test conditions: The temperature of the detection cell is 30±0.1°C, the gas flow rate is 120mL / min, the carrier gas used is high-purity nitrogen, a 10MHz QCM resonator, and gold electrodes with a diameter of 5mm on both sides.
[0041] Table 1. Frequency change and response H of QCM / C4mimCl response to different concentrations of ethanol i value.
[0042]
Embodiment 3
[0044] Using the same method in Example 1, ionic liquid C was prepared 4 mimPF 6 The QCM oscillator is a sensitive membrane material, at different temperatures, for different concentrations of CO 2 Gas was measured, at different temperatures, the resulting CO 2 in the ionic liquid C 4 mimPF 6 Henry's constant H in i See Table 2, with 1nH i Plot 1 / T ( Figure 4 shown), the resulting H i The relationship with temperature is ln H i = 10.30 - 1.95 × 10 3 × 1 T , Using the thermodynamic relational formula, the CO 2 Gases and Ionic Liquids C 4 mimPF 6 The enthalpy change ΔH of the action process (298K, 1atm) sol =-16.21±0.67 (kJ / mol), RSD=1.76%;
[0045] Free energy ΔG sol =9.32kJ / mol; entropy change ΔS sol =-85.63J / (mol·K).
[0046] Table 2. CO measured at different temper
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap