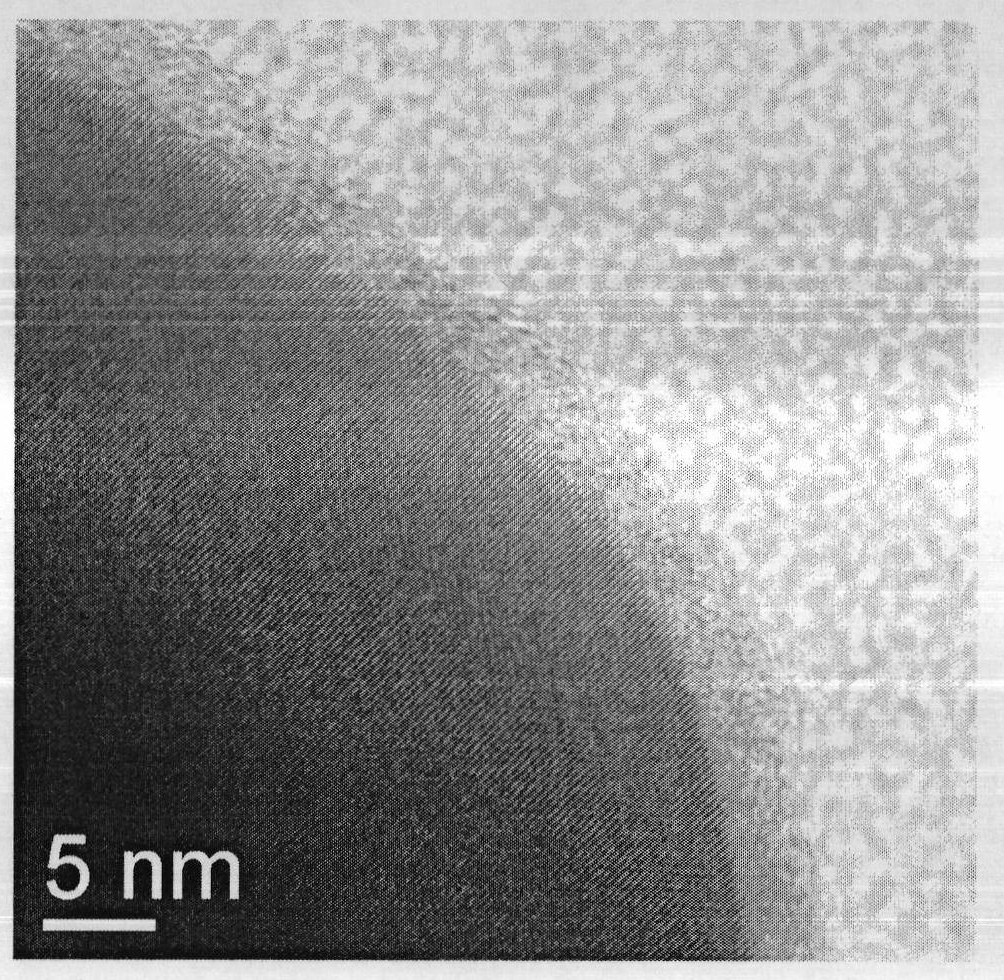
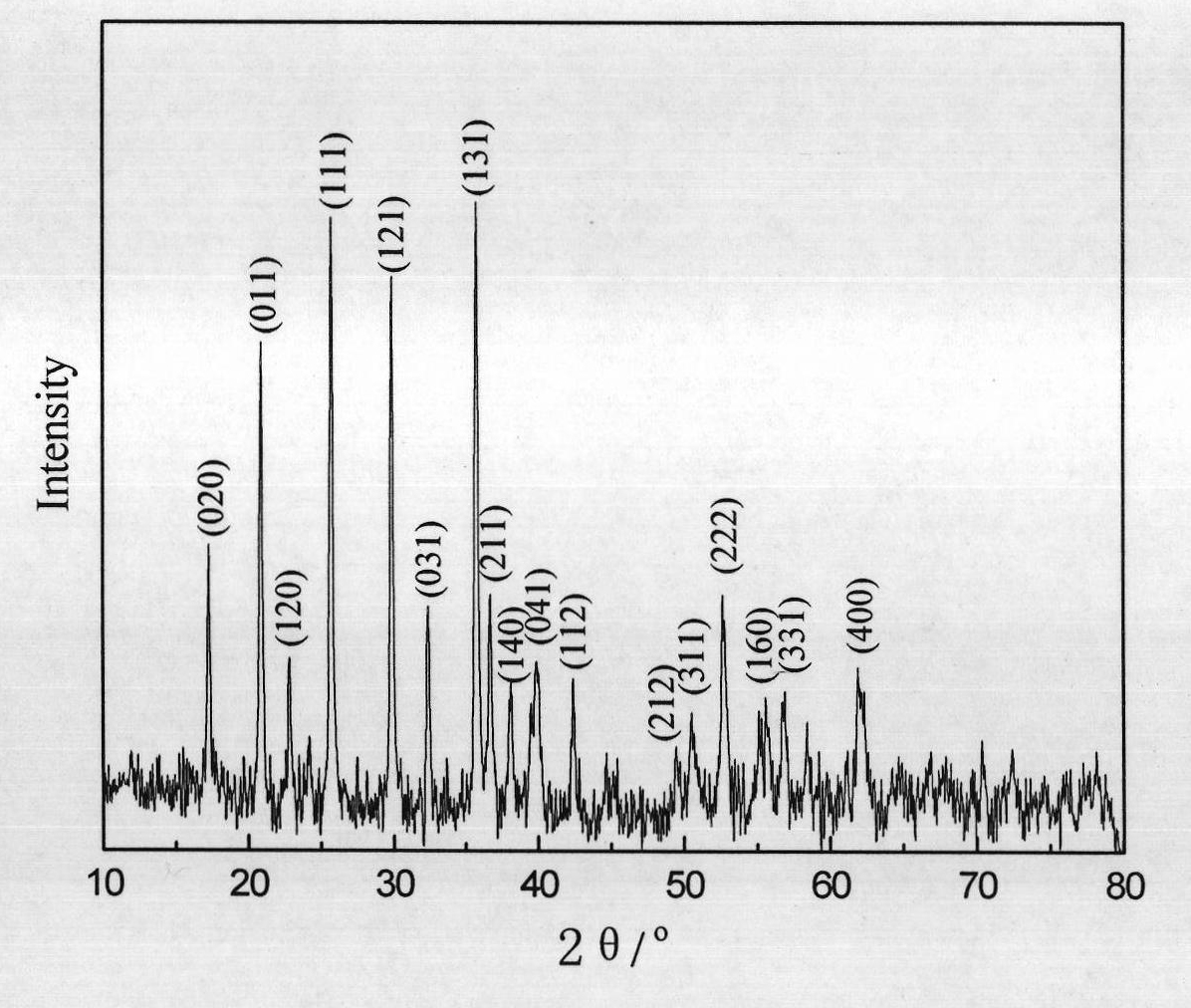
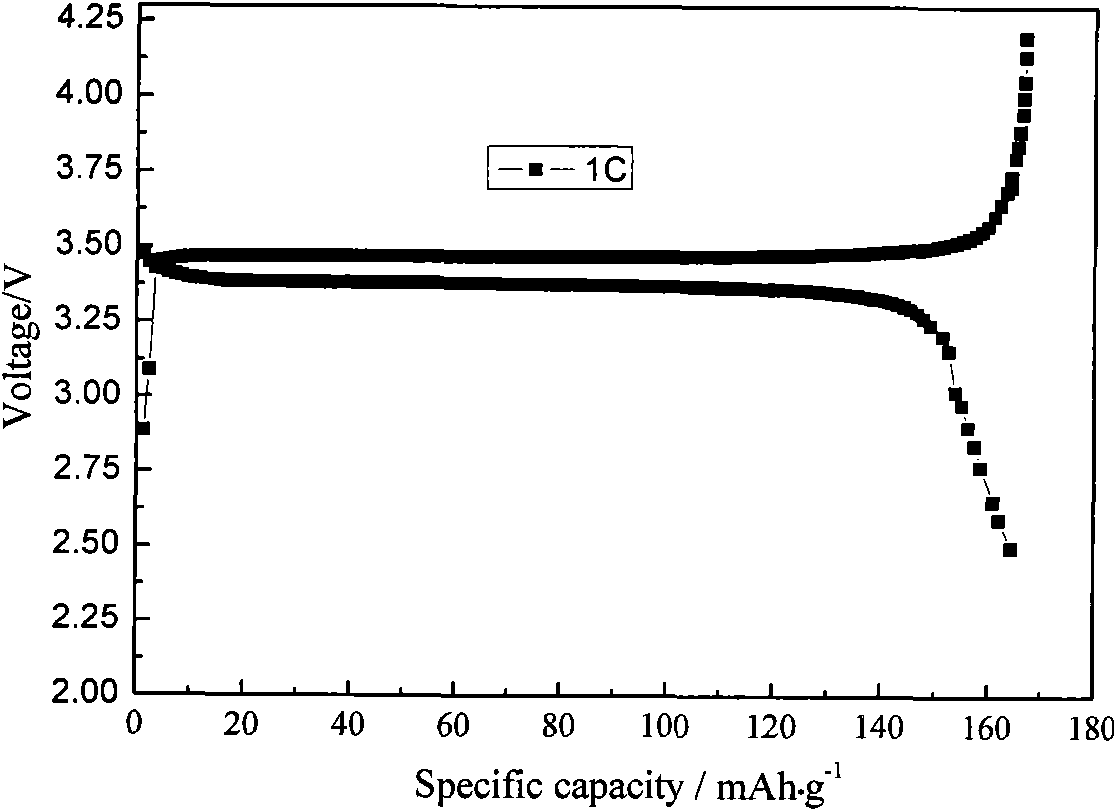
Method for realizing carbon coating of lithium iron phosphate through radio frequency plasma enhanced chemical vapor deposition
A radio frequency plasma, lithium iron phosphate technology, applied in the direction of electrical components, battery electrodes, circuits, etc., can solve the problems of material energy density reduction, long chemical reaction time, small carbon powder density, etc., to achieve short reaction time and easy thickness Control, the effect of large specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0026] Example 1:
[0027] Weigh 0.078mol each of lithium dihydrogen phosphate, ferrous oxalate and citric acid according to n(Li):n(Fe):n(P):n(citric acid)=1:1:1:1 (by mole), Dissolve citric acid in 250ml of deionized water, dissolve the iron source, lithium source and phosphorus source in 100ml of deionized water respectively, add dropwise to the citric acid solution, stir vigorously at room temperature to form a sol, and evaporate the sol to dryness in a water bath at 80°C The precursor gel was obtained, and the precursor gel was vacuum-dried at 100°C for 8 hours to obtain a dry gel, which was ball milled for 3 hours, and then placed in a tube furnace protected by argon, and pretreated at 300°C for 10 hours to remove CO 2 and H 2 O. Cool to room temperature with the furnace, ball mill for 2h. Calcined at 750°C for 10h in an argon atmosphere (heating rate 2°C·min -1 ), cooling and ball milling to obtain LiFePO 4 / C. The prepared sample LiFePO 4 / C placed in Shenyang
Example Embodiment
[0028] Example 2
[0029] Weigh lithium hydroxide, ferrous oxalate, ammonium dihydrogen phosphate and citric acid according to n(Li):n(Fe):n(P):n(citric acid)=1:1:1:1 (by mole) 0.078mol each, dissolve citric acid in 250ml deionized water, dissolve iron source, lithium source and phosphorus source in 100ml deionized water respectively, add dropwise to the citric acid solution, stir vigorously at room temperature to form a sol, steam in a water bath at 80°C After drying, a precursor gel was obtained, and the precursor gel was vacuum-dried at 100° C. for 8 hours to obtain a xerogel. Ball milled for 3 hours, placed in an argon-protected tube furnace, and pretreated at 300°C for 7 hours to remove CO 2 and H 2 O. Cool to room temperature with the furnace, ball mill for 2h. Calcined at 650°C for 20h in an argon atmosphere (heating rate 2°C·min -1 ), cooling and ball milling to obtain LiFePO 4 / C. The prepared sample LiFePO 4 / C placed in Shenyang Xinlantian Plasma Enhanced C
Example Embodiment
[0030] Example 3
[0031] Weigh lithium chloride, ferrous sulfate, ammonium dihydrogen phosphate and citric acid according to n(Li):n(Fe):n(P):n(citric acid)=1:1:1:1 (by mole) Each 0.078mol, citric acid was dissolved in 250ml of deionized water, iron source, lithium source and phosphorus source were dissolved in 100ml of deionized water, respectively, added to the citric acid solution, stirred vigorously at room temperature to form a sol. The precursor gel was obtained after evaporating to dryness in a water bath at 80° C., and dried in vacuum at 100° C. for 8 hours to obtain a xerogel. Ball milled for 3 hours, placed in an argon-protected tube furnace, pretreated at 300°C for 8 hours, to remove CO 2 and H 2 O. Cool to room temperature with the furnace, ball mill for 2h. Calcined at 800 °C for 10 h in an argon atmosphere (heating rate 2 °C min -1 ), cooling and ball milling to obtain LiFePO 4 / C. The prepared sample LiFePO 4 / C placed in Shenyang Xinlantian Plasma Enh
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap