Method for improving thermal stability of glucose oxidase
A technology for glucose oxidase and stability, applied in the field of enzyme engineering, can solve the problems of reducing and increasing the thermal stability of fusion enzymes, and achieve the effects of improving thermal stability, convenient application, and improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
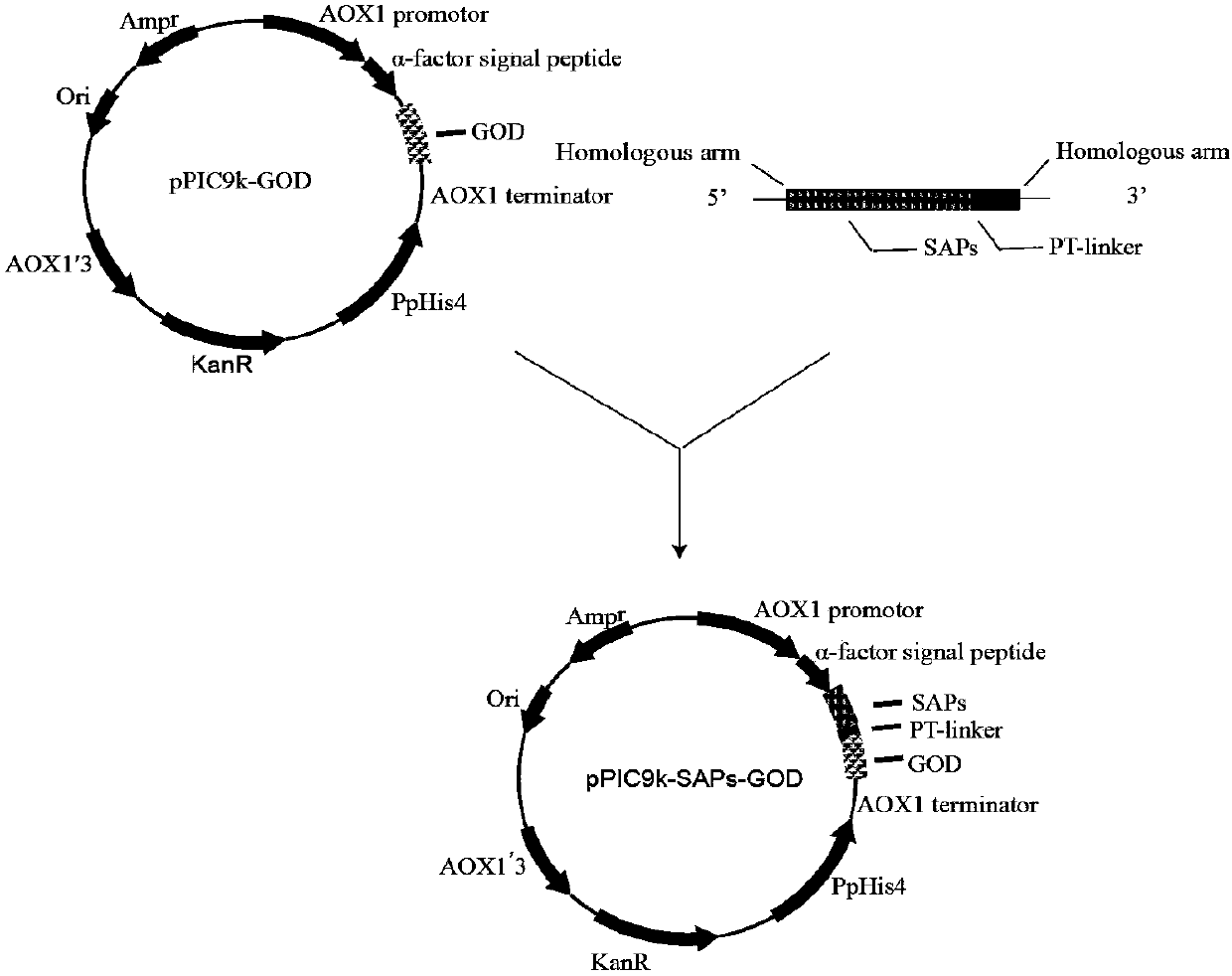
[0038] Embodiment 1: Construction of recombinant fusion strain
[0039] The first step is the acquisition of parental short peptide genes
[0040] The amino acid sequences of the parental short peptides and short peptides are respectively, the amino acid sequence of short peptide 1 is shown in SEQ ID NO.1; the amino acid sequence of short peptide 2 is shown in SEQ ID NO.2, and the amino acid sequence of short peptide 3 is shown in SEQ ID NO. .3, the amino acid sequence of short peptide 4 is shown in SEQ ID NO.4, the amino acid sequence of short peptide 5 is shown in SEQ ID NO.5, the amino acid sequence of short peptide 6 is shown in SEQ ID NO.6, and the amino acid sequence of short peptide 7 is shown in SEQ ID NO.6 The sequence is shown in SEQ ID NO.7. Amplification was performed using the primers in the table below. The underlined part is the sequence homologous to the recombinant plasmid pPIC9k-GOD constructed in our laboratory, which is called the homology arm.
[0041] Tab
Embodiment 2
[0048] Example 2: Purification of enzymes produced by fermentation of recombinant fusion strains
[0049] (1) Preparation and purification of crude enzyme solution
[0050] The method steps of bacterial strain expressing glucose oxidase through culture:
[0051] Pick 25 transformants on the MD plate after transformation and put them on the YPD plate containing G418 resistance (G418 resistance concentration gradient is 1mg / mL, 2mg / mL, 3mg / mL, 4mg / mL), pick 4mg / ml 5-10 recombinant bacteria on the resistant YPD plate were subjected to shake flask fermentation.
[0052] Seed culture: Pick a single colony on the YPD plate and put it in 25mLYPD liquid medium (250ml shake flask) and culture it at 30°C and 220r / min for 18-20h.
[0053] Shake flask fermentation: Add 10% of the seed liquid into 50ml BMGY medium (500ml shake flask), culture at 30°C and 220r / min for 24h, centrifuge all the bacteria and wash twice with an equal volume of normal saline, centrifuge , Transfer a
Embodiment 3
[0055] Embodiment 3: The determination of the activity of glucose oxidase and the thermostability comparison of fusion enzyme and initial enzyme
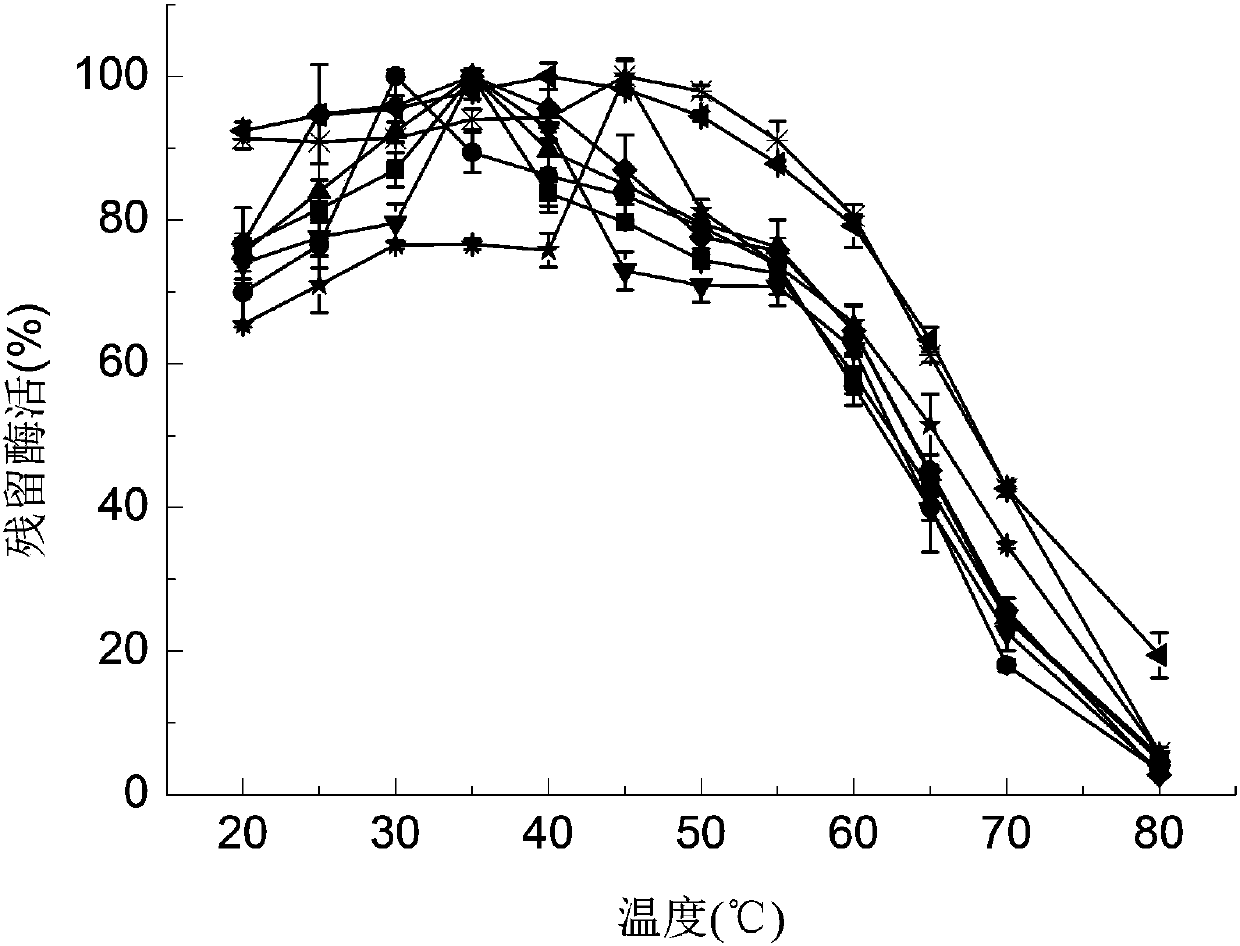
[0056] Determination of the optimum reaction temperature: put the purified GOD enzyme solution in the same assay system, at different temperatures
[0057] (20°C, 25°C, 30°C, 35°C, 40°C, 45°C, 50°C, 55°C, 60°C, 65°C, 70°C, 80°C) conditions, the enzyme activity was measured. And take the highest enzyme activity value as 100%.
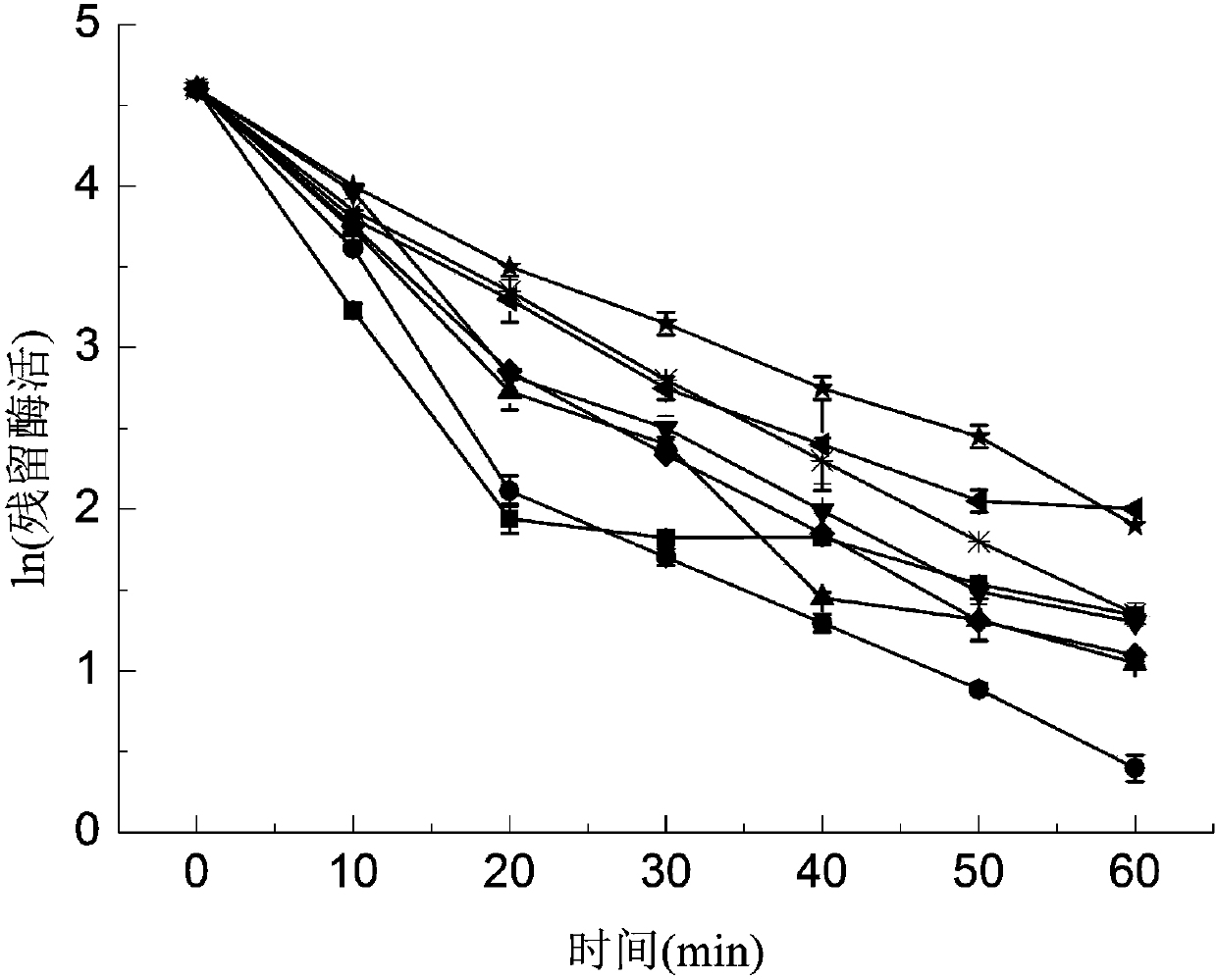
[0058] Measurement of temperature stability: The purified GOD enzyme solution was heated at 60°C for 60 minutes, and its relative enzyme activity was measured every 10 minutes. The enzyme activity without heat bath treatment was regarded as 100%.
[0059] Such as figure 1 As shown, the optimum temperature of fusion enzyme 1 is 45 °C, the optimum temperature of fusion enzyme 2, fusion enzyme 3, fusion enzyme 4 and fusion enzyme 5 and the initial enzyme are all 35 °C, and the optimum temperature of fusion enzyme
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap