Method for preparing hemostatic material based on air jet pulverization technology
A hemostatic material and jet pulverization technology, applied in the field of biomedical hemostatic materials, can solve the problems of inconvenient clinical use, complex preparation process, poor hemostatic effect, etc., and achieve significant technical and clinical advantages, simple preparation process, and excellent hemostatic effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
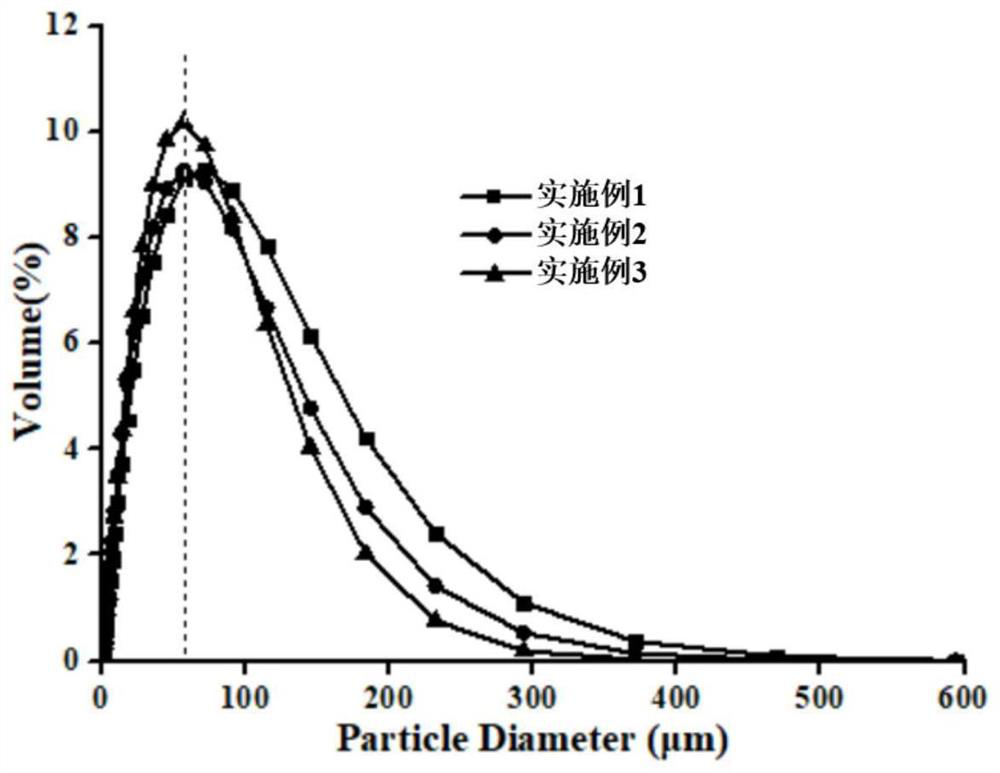
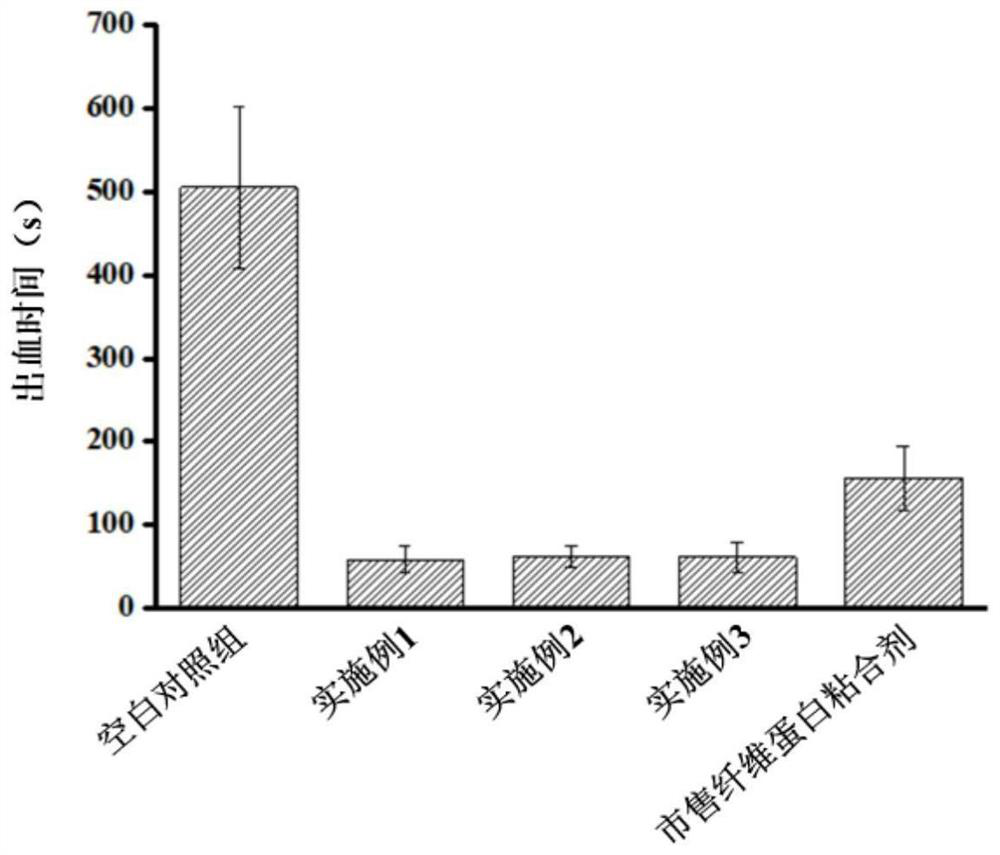
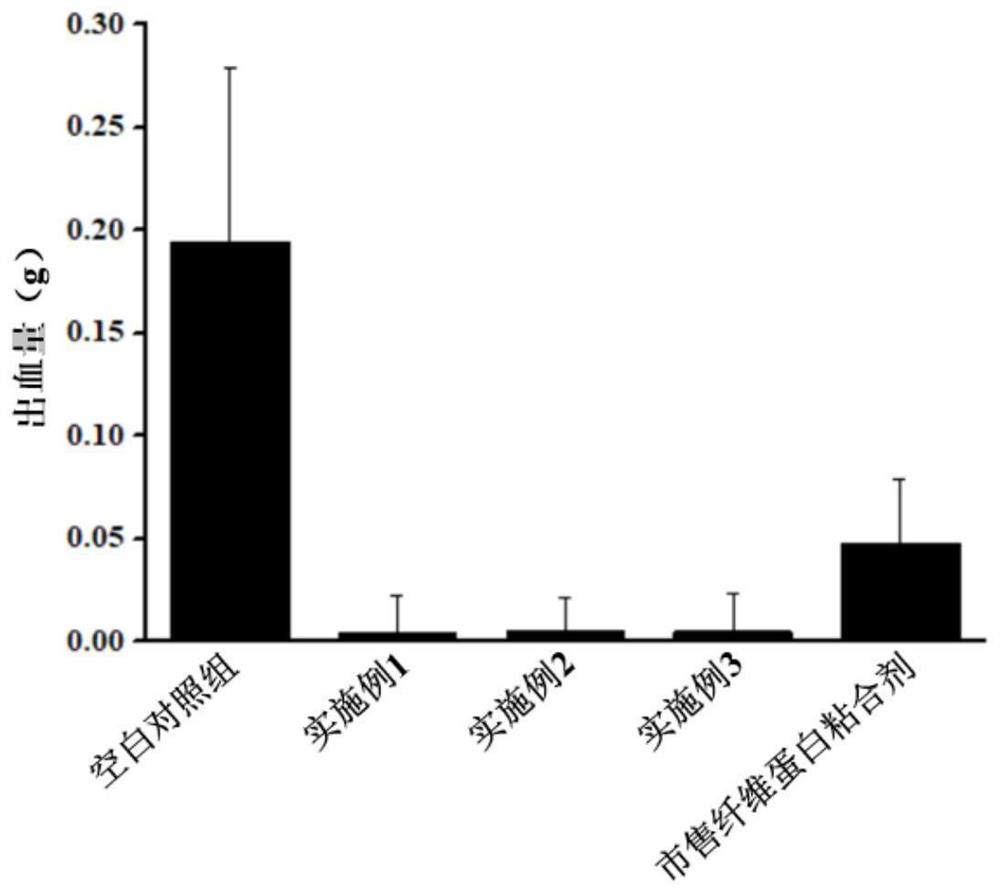
Embodiment 1
[0034] A method for preparing a hemostatic material based on jet milling technology, comprising the steps of:
[0035] One, the preparation of fibrinogen powder:
[0036] (1) Prepare fibrinogen solution: add fibrinogen, trehalose, glycine, sodium chloride, sodium citrate and arginine hydrochloride to water for injection, and stir evenly to obtain the fibrinogen solution; The mass percent of each component in the fibrinogen solution is: 2.5wt% of fibrinogen, 10wt% of trehalose, 2wt% of glycine, 1.0wt% of sodium chloride, 0.1wt% of sodium citrate, 2.0wt% of arginine hydrochloride %, the balance is water for injection;
[0037] (2) Preparation of fibrinogen powder: the fibrinogen solution is placed in a freeze-drying tray, vacuum freeze-dried, and then pulverized by an ultrafine powder jet mill to obtain a fibrinogen powder; the vacuum freeze-dry The process includes: pre-freezing at -55°C for 8 hours, then drying at -5°C for 55 hours, and then secondary drying at 35°C for 12 hour
Embodiment 2
[0044] A method for preparing a hemostatic material based on jet milling technology, comprising the steps of:
[0045] One, the preparation of fibrinogen powder:
[0046] (1) Prepare fibrinogen solution: add fibrinogen, trehalose, glycine, sodium chloride, sodium citrate and arginine hydrochloride to water for injection, and stir evenly to obtain the fibrinogen solution; The mass percent of each component in the fibrinogen solution is: 3.0wt% of fibrinogen, 15wt% of trehalose, 6wt% of glycine, 1.25wt% of sodium chloride, 0.35wt% of sodium citrate, 2.25wt% of arginine hydrochloride %, the balance is water for injection;
[0047] (2) Preparation of fibrinogen powder: the fibrinogen solution is placed in a freeze-drying tray, vacuum freeze-dried, and then pulverized by an ultrafine powder jet mill to obtain a fibrinogen powder; the vacuum freeze-dry The process includes: pre-freezing at -35°C for 12 hours, then drying at -10°C for 50 hours, and then secondary drying at 25°C for 15
Embodiment 3
[0054] A method for preparing a hemostatic material based on jet milling technology, comprising the steps of:
[0055] One, the preparation of fibrinogen powder:
[0056] (1) Prepare fibrinogen solution: add fibrinogen, trehalose, glycine, sodium chloride, sodium citrate and arginine hydrochloride to water for injection, and stir evenly to obtain the fibrinogen solution; The mass percent of each component in the fibrinogen solution is: 3.5wt% of fibrinogen, 20wt% of trehalose, 10wt% of glycine, 1.5wt% of sodium chloride, 0.6wt% of sodium citrate, 2.5wt% of arginine hydrochloride %, the balance is water for injection;
[0057] (2) Preparation of fibrinogen powder: the fibrinogen solution is placed in a freeze-drying tray, vacuum freeze-dried, and then pulverized by an ultrafine powder jet mill to obtain a fibrinogen powder; the vacuum freeze-dry The process includes: pre-freezing at -20°C for 15 hours, then drying at 0°C for 65 hours, and then secondary drying at 30°C for 10 hou
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap