Amino acid sequence of anti-okadaic acid single-chain antibody and expression vector thereof
A technology of Datian haisalic acid and single-chain antibody, which is applied in the direction of anti-animal/human immunoglobulin, introduction of foreign genetic material using carrier, polypeptide containing affinity tag, etc. Mass production and other problems, to avoid animal dependence and difficulty in antibody affinity maturation, efficient preparation, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
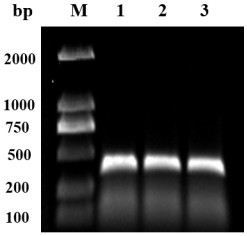
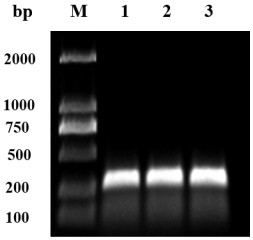
[0028] Example 1 Preparation of OA-scFv gene
[0029] 1.1 Total RNA extraction and cDNA synthesis.
[0030] The preparation method of anti-OA monoclonal antibody hybridoma cells is the same as the reference Wang R , Zengl L , Yang H, et al. Detection of okadaic acid (OA) using ELISA and colloidal goldimmunoassay based on monoclonal antibody.[J]. Journal of Hazardous Materials, 2017, 339(oct.5):154-160. The total RNA of anti-OA monoclonal antibody hybridoma cells was extracted using ER501-01 kit (Beijing Quanshijin Biotechnology Co., Ltd.). Using the extracted total RNA as a template, cDNA was synthesized using the K1691 cDNA Synthesis Kit (Thermo Fisher Scientific (China) Co., Ltd.). All operations were performed according to the kit instructions.
[0031] 1.2 Design of primers
[0032] The designed primers are shown in Table 2 and were synthesized by Jinweizhi Company.
[0033] Table 2 Primer sequence list
[0034]
[0035] Note: M=A / C, R=A / G, S=C / G, W=A / T
[0
Example Embodiment
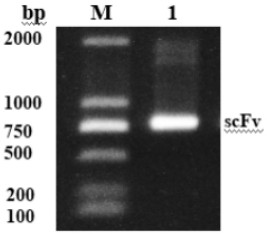
[0056] Example 2 pET-MBP-His-OA-scFv expression vector construction and expression identification
[0057] 2.1 Construction of pET-MBP-His-OA-scFv expression vector
[0058] The OA-ScFv gene was used EcoR I and Hind Ⅲ After the double digestion, use agarose gel electrophoresis, and use the gel recovery kit (purchased from ThermoFisher Scientific) to recover the digested products. The pET28a-MBP-T7-6×His vector (purchased from Wuhan Biotechnology Co., Ltd.) containing soluble tag maltose binding protein was also passed through EcoR I and Hind Ⅲ After double enzyme digestion, use agarose gel electrophoresis to recover large fragments. The OA-ScFv gene digestion product fragment was ligated with the pET28a-MBP-T7-6×His vector that had undergone the same double digestion at 16°C for 2 hours (or overnight at 4°C) to construct the recombinant plasmid pET-MBP-His -OA-scFv.
[0059] 2.2 Transformation of recombinant plasmids and expression and purification of recombinant prot
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap