Conductive polymers having highly enhanced solubility in organic solvent and synthesizing process thereof
A technology of conductive polymers and organic solvents, which can be used in household utensils, mirrors, picture frames, etc., and can solve problems such as oxidants and pyrrole monomers that are difficult to dissolve in the same solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
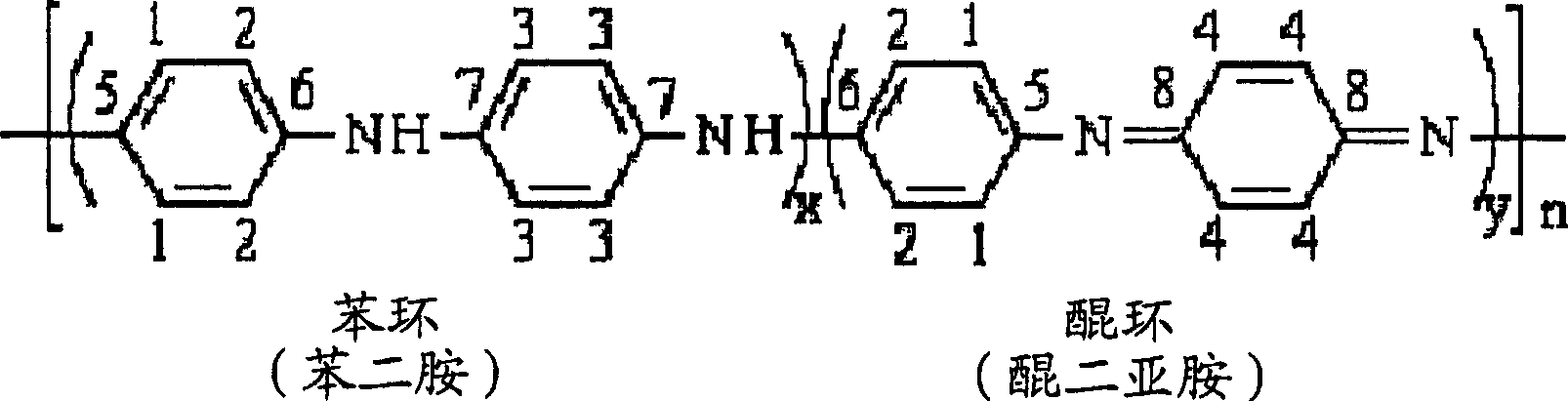
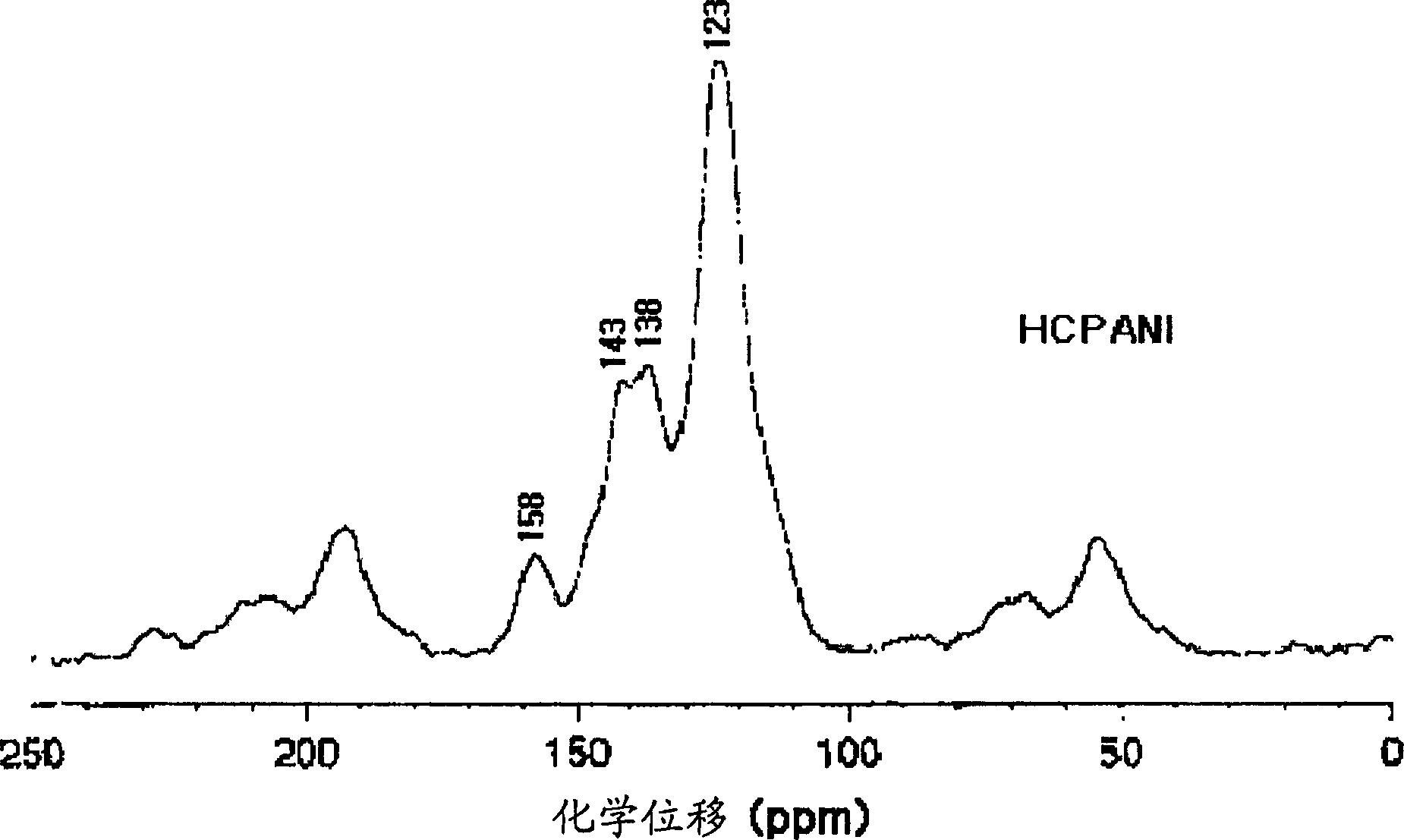
[0144] Embodiment 1: the preparation of high conductivity polyaniline (HCPANI)
[0145] In this example the emeraldine base form (EB) of high conductivity polyaniline (HCPANI) was prepared. 100 ml of distilled and purified aniline was slowly added dropwise to 6 liters of 1 mol / liter hydrochloric acid, and then the obtained solution was mixed with 4 liters of isopropanol. The temperature of the mixed solution was kept at -15°C. Ammonium persulfate ((NH 4 ) 2 S 2 o 8 ) 56 g was dissolved in 2 liters of 1 mol / liter of hydrochloric acid to form a solution, and then this solution was slowly added dropwise to the above-mentioned mixed solution under stirring to initiate polymerization, and the time for dropping was 40 minutes. After 3 hours, the polymerization was complete and a precipitate formed. The obtained precipitate was filtered with filter paper, and then washed with 1 liter of 1 mol / liter ammonium hydroxide (NH 4 OH) solution for washing. The precipitate was move
Embodiment 2
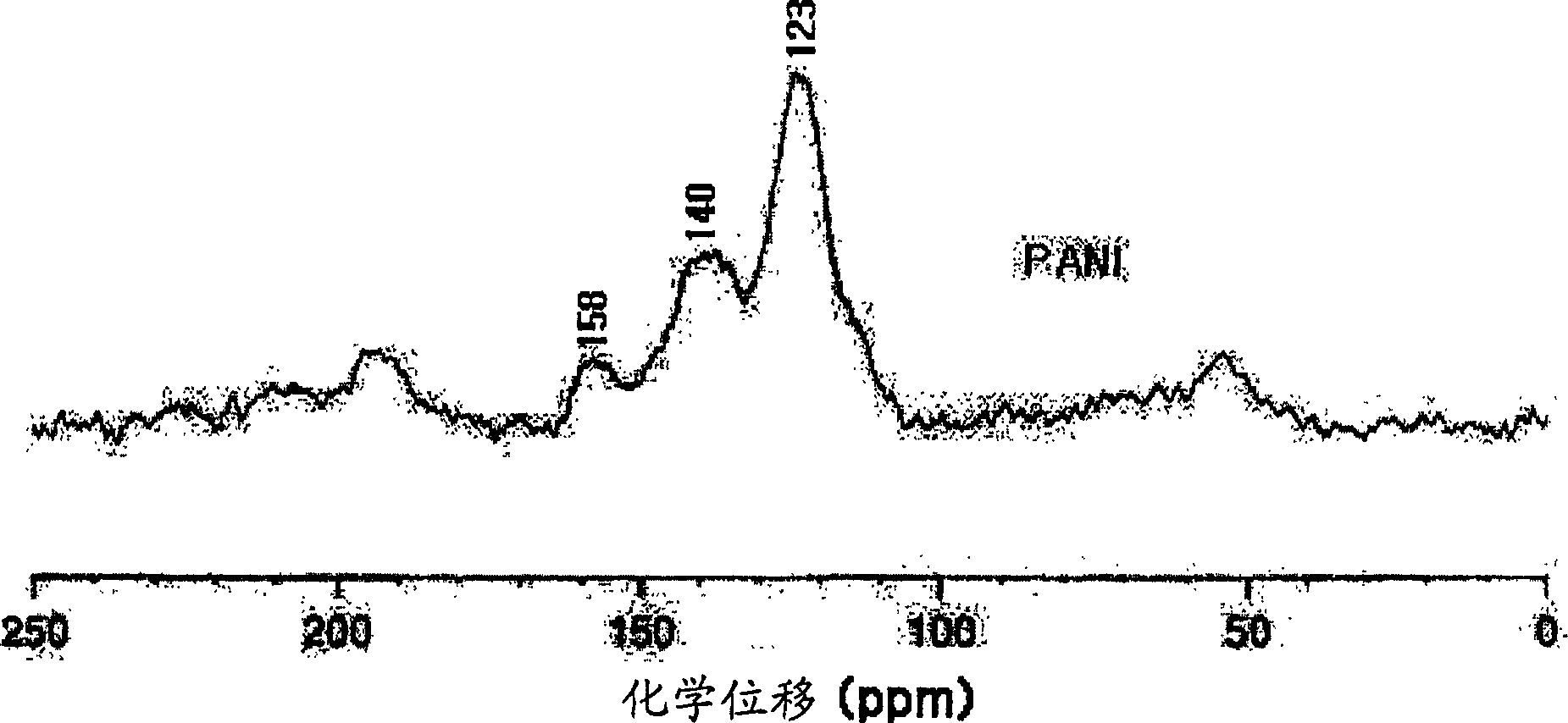
[0149] Except that the polymerization reaction temperature was about -25°C, other steps and conditions were the same as in Example 1. The polymerization reaction is carried out for 4-6 hours. It was determined by infrared spectroscopy and NMR analysis (results not shown) that the synthesized substance was emeraldine base polyaniline.
Embodiment 3
[0151] Except that the time for adding the free radical initiator ammonium persulfate was 3 hours, other steps and conditions were the same as in Example 1. The polymerization reaction is carried out for 3 to 8 hours. It was determined by infrared spectroscopy and NMR analysis (results not shown) that the synthesized substance was emeraldine base polyaniline.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent density | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap