Starch esterification method
a starch esterification and starch technology, applied in the direction of bulk chemical production, etc., can solve the problems of not forming true solutions, unable to achieve the effect of reducing the number of acetylated starches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
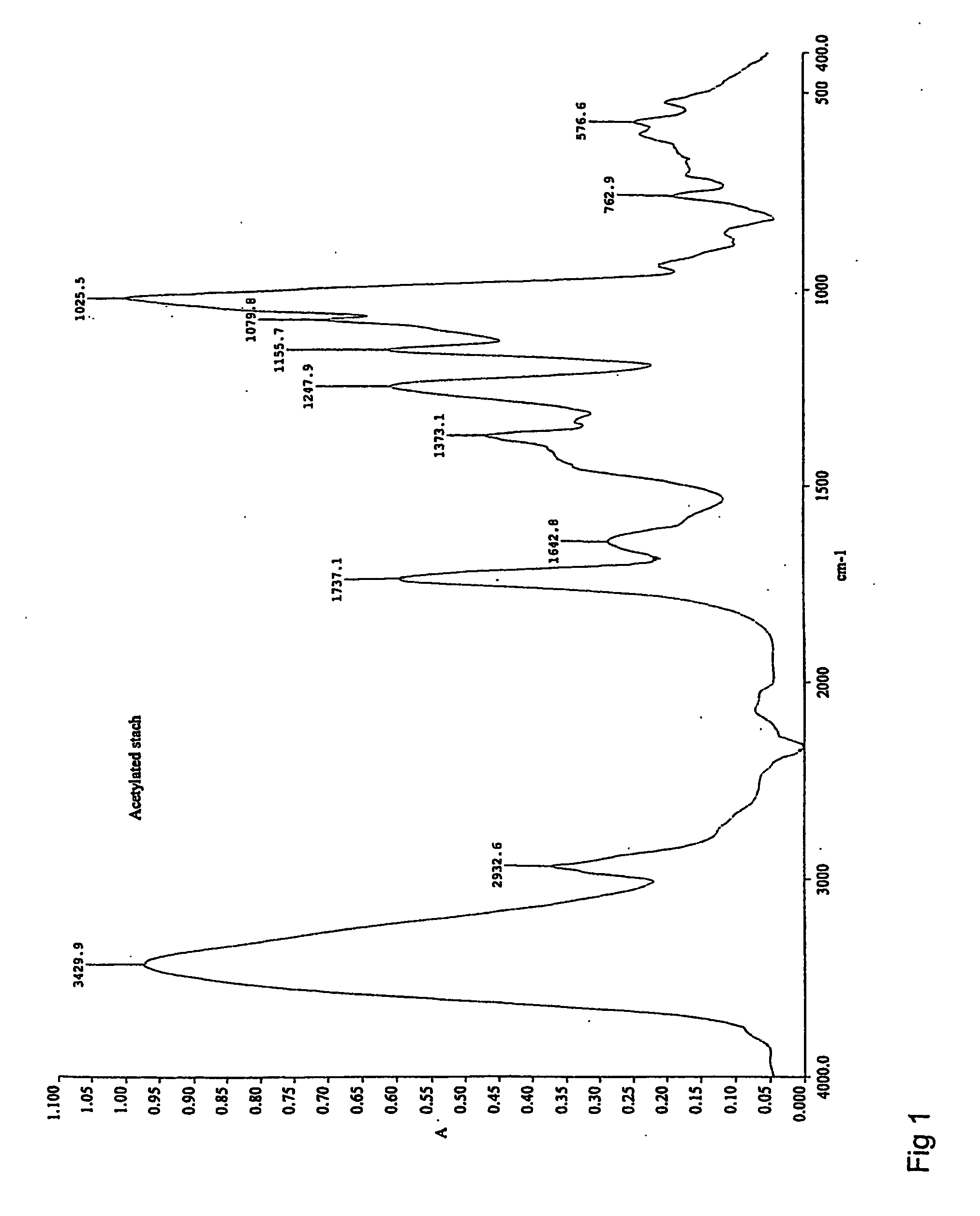
[0095] Acetylation of Native Barley Starch
[0096] A 150 mg (1 mmol) sample of oven dried native barley starch was added into ionic liquid (BMIMCl, 3 ml) and the resulting mixture was stirred at 75° C. for 25 minutes. A clear, 5% starch solution was formed and acetic anhydride (0.3 ml, 3 mmol) was added therein. The reaction was conducted for 15 minutes and quenched by adding dry ethanol (3 ml) with a syringe into the reaction mixture. While stirring the resulting mixture, the product precipitated from the reaction medium. The product was filtered off and washed with dry ethanol, oven dried and analyzed with FTIR. The obtained spectrum for acetylated starch is shown in FIG. 1 showing the acetyl OAc peak at 1737.1 cm−1.
example 2
[0097] Acetylation of Hydrolyzed Starch
[0098] A 150 mg (1 mmol) sample of oven dried, hydrolyzed starch was added into ionic liquid (BMIMCl, 3 ml) and the resulting mixture was stirred at 75° C. for 25 minutes. A clear, 5% starch solution was formed and acetic anhydride (0.3 ml, 3 mmol) was added therein. The reaction was conducted for 15 minutes and quenched by adding dry isopropanol (3 ml) with a syringe into the reaction mixture. While stirring the resulting mixture, the product precipitated from the reaction medium. The product was filtered off and washed with dry isopropanol, oven dried and analyzed with FTIR. The spectrum was in accordance with the one shown in FIG. 1.
example 3
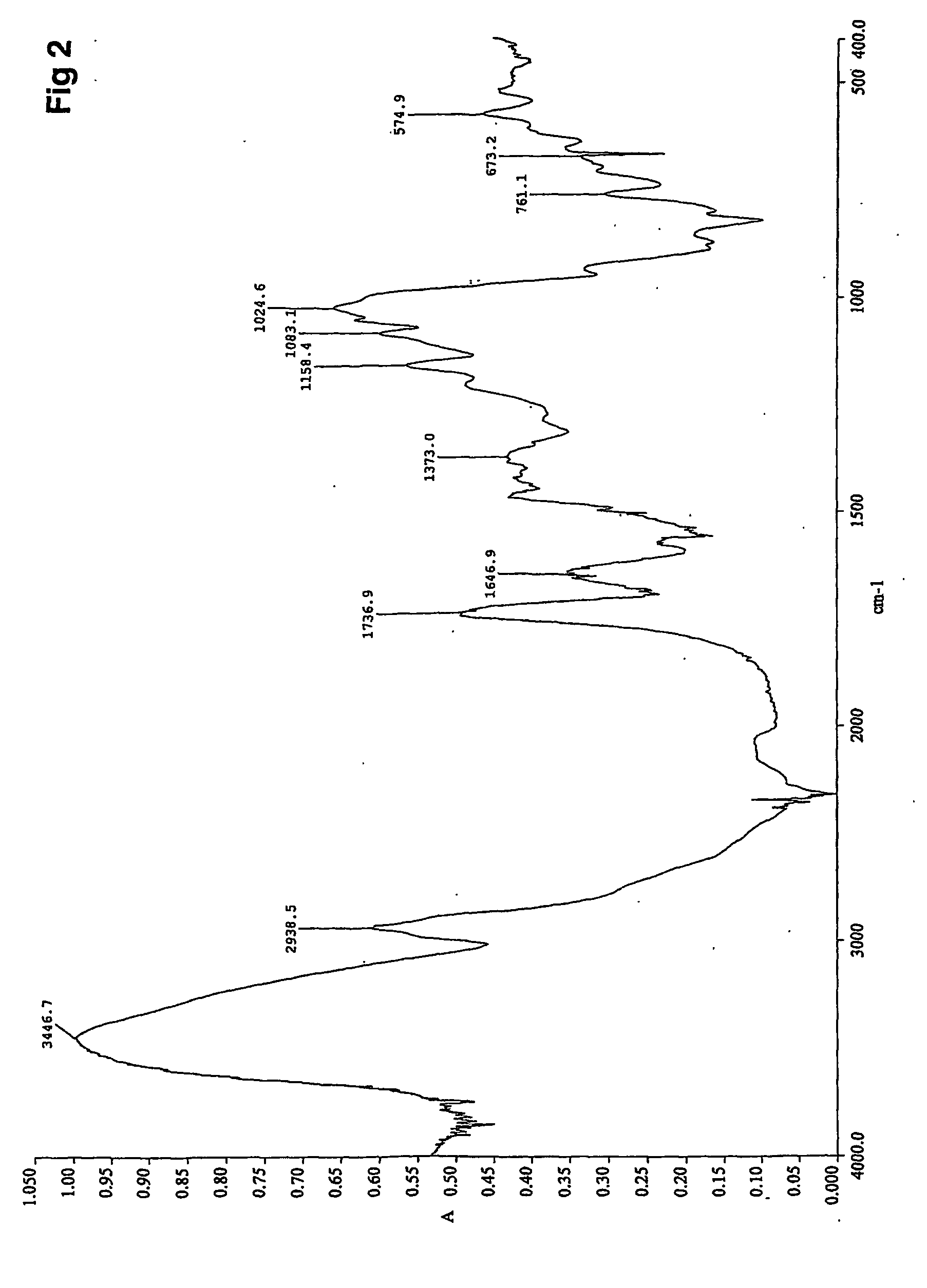
[0099] Propionylation of Native Barley Starch, D.S.=1
[0100] A 2 g (12.4 mmol) sample of oven dried native barley starch was added into ionic liquid (BMIMCl, 20 g) and the resulting mixture was stirred at 80° C. for 20 minutes. A faintly opaque, 10% starch solution was formed and propionic anhydride (1.6 ml, 12.4 mmol) was slowly added therein. The reaction was conducted for 2 hours and quenched by adding ethanol (30 ml) into the reaction mixture. While stirring the resulting mixture, the product precipitated from the reaction medium. The product was filtered off and washed with ethanol, oven dried and analyzed with FTIR. The obtained spectrum for propionylated starch is shown in FIG. 2 showing the O-propionyl peak at 1736.9 cm−1. The prepared propionylated starch was soluble in water, but insoluble in alcohols such as methanol, ethanol and isopropanol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap