Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4 results about "Carnitine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carnitine (β-hydroxy-γ-N-trimethylaminobutyric acid, 3-hydroxy-4-N,N,N-trimethylaminobutyrate) is a quaternary ammonium compound involved in metabolism in most mammals, plants, and some bacteria. Carnitine may exist in two isomers, labeled D-carnitine and L-carnitine, which are both biologically active. At room temperature, pure carnitine is a white powder, and a water-soluble zwitterion with low toxicity. Carnitine only exists in animals as the L-enantiomer, and D-carnitine is toxic because it inhibits the activity of L-carnitine. Carnitine, derived from an amino acid, is found in nearly all organisms and animal tissue. Carnitine is the generic expression for a number of compounds that include L-carnitine, acetyl-L-carnitine, and propionyl-L-carnitine. It is most accumulated in cardiac and skeletal muscles as it accounts for 0.1% of its dry matter. It was first derived from meat extracts in 1905, therefore the name carnitine is derived from Latin "carnus" or flesh. The body synthesizes enough carnitine from lysine side chains to keep up with the needs of energy production in the body as carnitine acts as a transporter of long-chain fatty acids into the mitochondria to be oxidized and produce energy. Some individuals with genetic or medical disorders (such as preterm infants) cannot make enough, so this makes carnitine a conditionally essential nutrient for them.
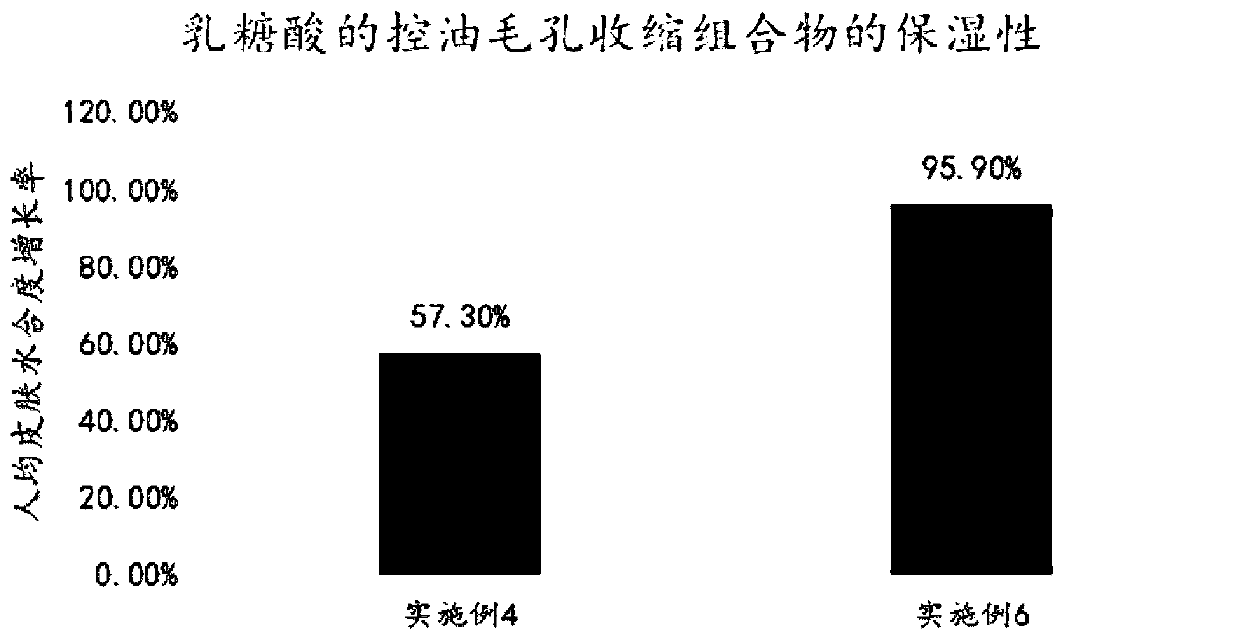
Oil-control pore-shrinking composition containing lactobionic acid and application thereof
InactiveCN111346027AInhibit abnormal oil productionPromote new lifeCosmetic preparationsToilet preparationsBiologyNicotinamide
Owner:GUANGZHOU PINYU BEAUTY INNOVATION TECH CO LTD
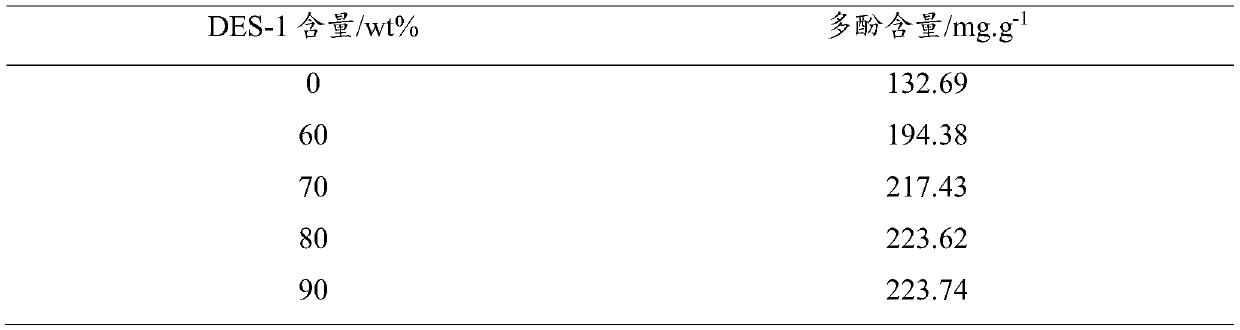
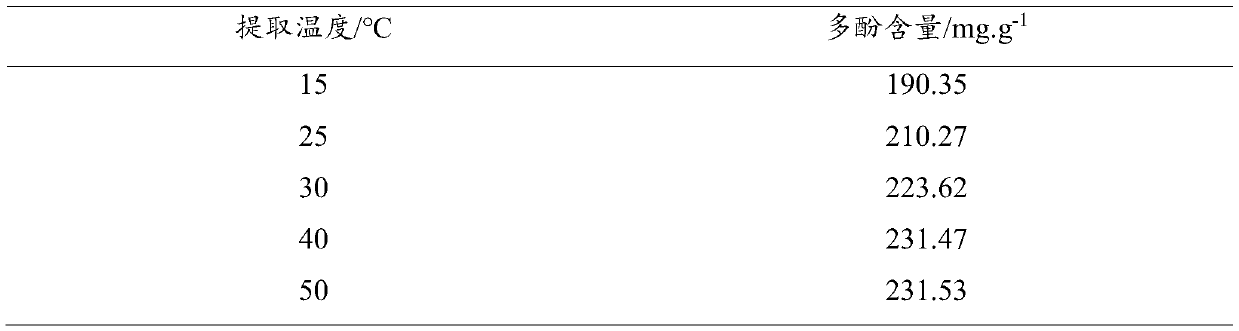
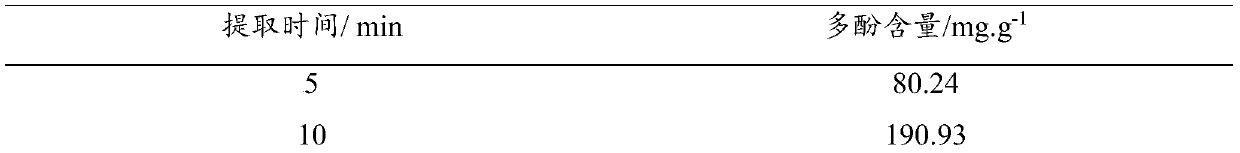
Preparation method of L-carnitine amino acid deep eutectic solvent and application thereof in polyphenol extraction
ActiveCN110862328AImprove extraction efficiencyIncrease contentOrganic compound preparationAmino-carboxyl compound preparationPhysical chemistrySolvent
Owner:中科萱嘉医养(珠海)健康科技有限公司
Dried blood spot quality control product for neonatal screening and preparation method thereof
PendingCN114076701ASimple preparation processEasy to operateComponent separationPreparing sample for investigationFull Term NeonateFilter paper
The invention discloses a dried blood spot quality control product for neonatal screening and a preparation method thereof. The dried blood spot quality control product comprises a filter paper carrier, the filter paper carrier is infiltrated with a blood working solution, and the blood working solution takes Chinese species blood as a matrix solution, and is formed by mixing the matrix solution and an extracting solution according to a certain proportion. The extracting solution comprises amino acid, free acyl carnitine, lysophosphatidylcholine, orotic acid and succinylacetone. The dried blood spot quality control product disclosed by the invention has good uniformity and stability, and can be used as an indoor quality control product in a neonatal screening project; and the preparation process is simple and convenient to operate.
Owner:SHANGHAI ANPEL SCI INSTR
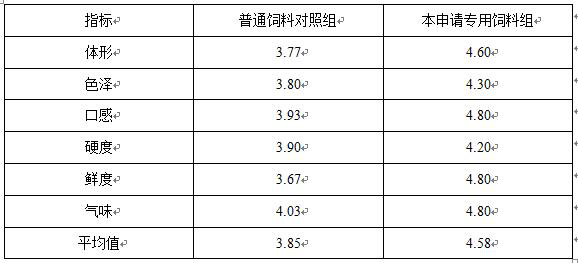
Special feed for factory-like circulating water short-term grass carp breeding and preparation method
InactiveCN113558154AEasy to shapeExcellent muscle freshnessAnimal feeding stuffAccessory food factorsBiotechnologyInosinic acid
Owner:宝应新佳鱼水产科技有限公司
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap