Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4 results about "Dextran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dextran is a complex branched glucan (polysaccharide derived from the condensation of glucose). IUPAC defines dextrans as "Branched poly-α-d-glucosides of microbial origin having glycosidic bonds predominantly C-1 → C-6". Dextran chains are of varying lengths (from 3 to 2000 kilodaltons).
Antigen-specific T lymphocyte freezing medium and preparation method and application thereof
ActiveCN107148967AReduce operating proceduresLow pollution rateDead animal preservationSodium Chloride InjectionT lymphocyte
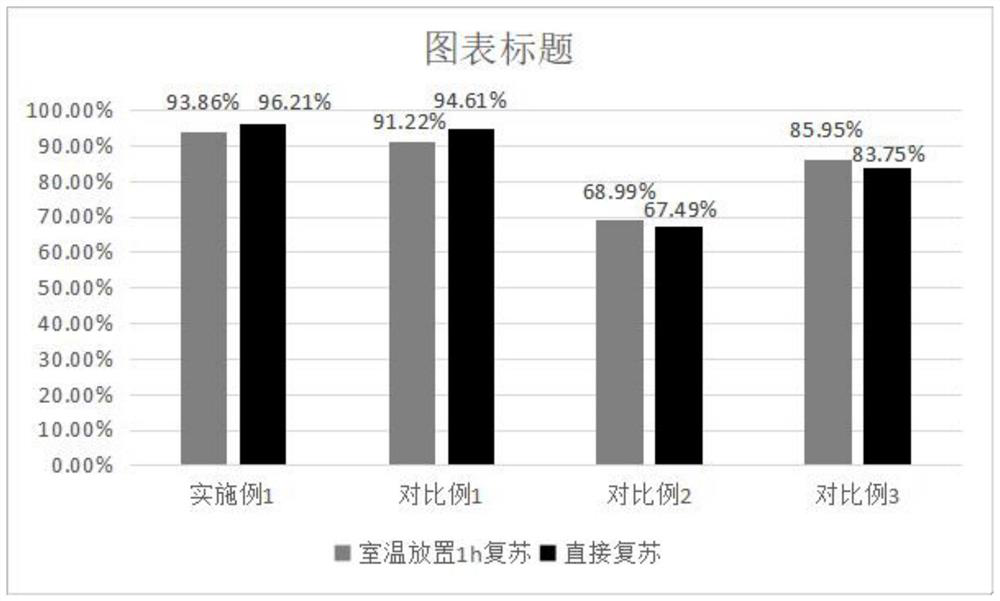
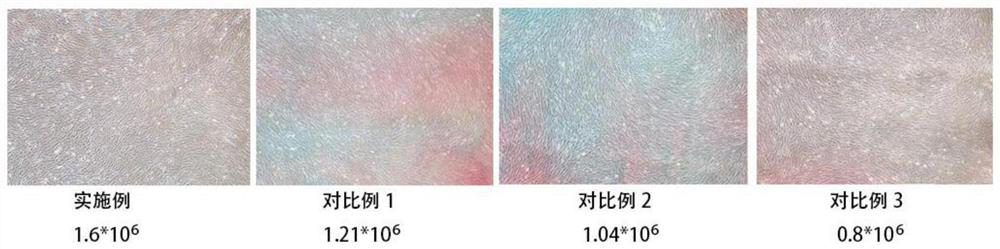
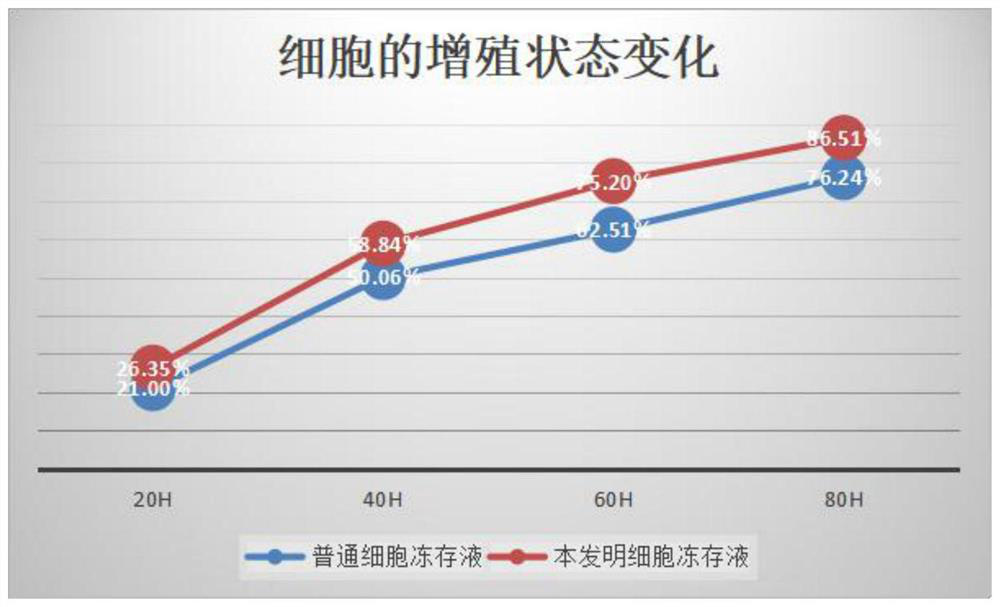
The invention provides an antigen-specific T lymphocyte freezing medium, comprising freezing medium A and freezing medium B; the freezing medium A includes, by volume, 30-40% of plasmalyte electrolyte injection, 30-40% of glucose and sodium chloride injection, 5-15% of dextran glucose injection and 15-25% of human albumin solution; the freezing medium B includes, by volume, 20-30% of plasmalyte electrolyte injection, 20-30% of glucose and sodium chloride injection, 5-15% of dextran glucose injection, 15-25% of human albumin solution and 10-20% of dimethyl sulfoxide; the freezing medium A and the freezing medium B are stored separately; in use, the freezing medium A and the freezing medium B are mixed in a ratio of 1:(0.5-2), forming the antigen-specific T lymphocyte freezing medium. The antigen-specific T lymphocyte freezing medium is capable of enabling less crystal to form in cells, increasing cell survival rate and maintaining tumor-killing ability of cells. The invention also provides a preparation method and application of the antigen-specific T lymphocyte freezing medium and antigen-specific T lymphocyte injection.
Owner:CHENZHOU BINZE MEDICAL LAB CO LTD
An antibacterial whitening mouthwash and a preparation method thereof
InactiveCN109172475AImprove securityHigh antibacterial activityCosmetic preparationsAntibacterial agentsManihotAdditive ingredient
Owner:上海怡竹生物科技有限公司
Novel serum-free cell freezing medium formula
InactiveCN114467924AImprove survival rateHigh activityDead animal preservationSerum freeCryopreservation
Owner:杭州原生生物科技有限公司
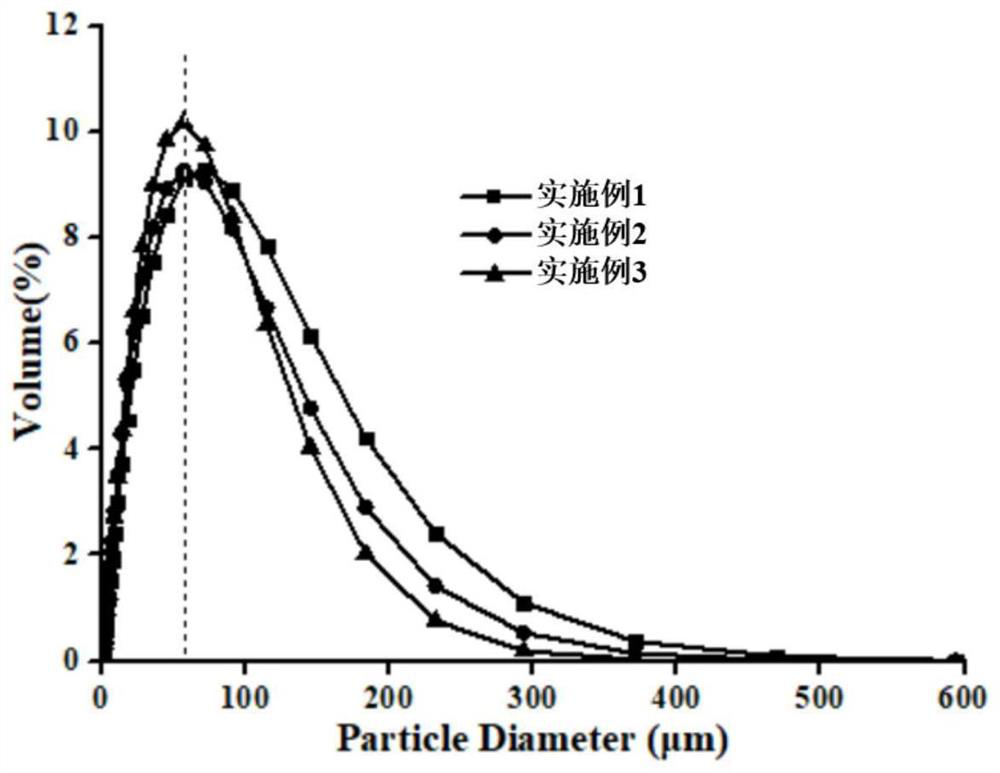
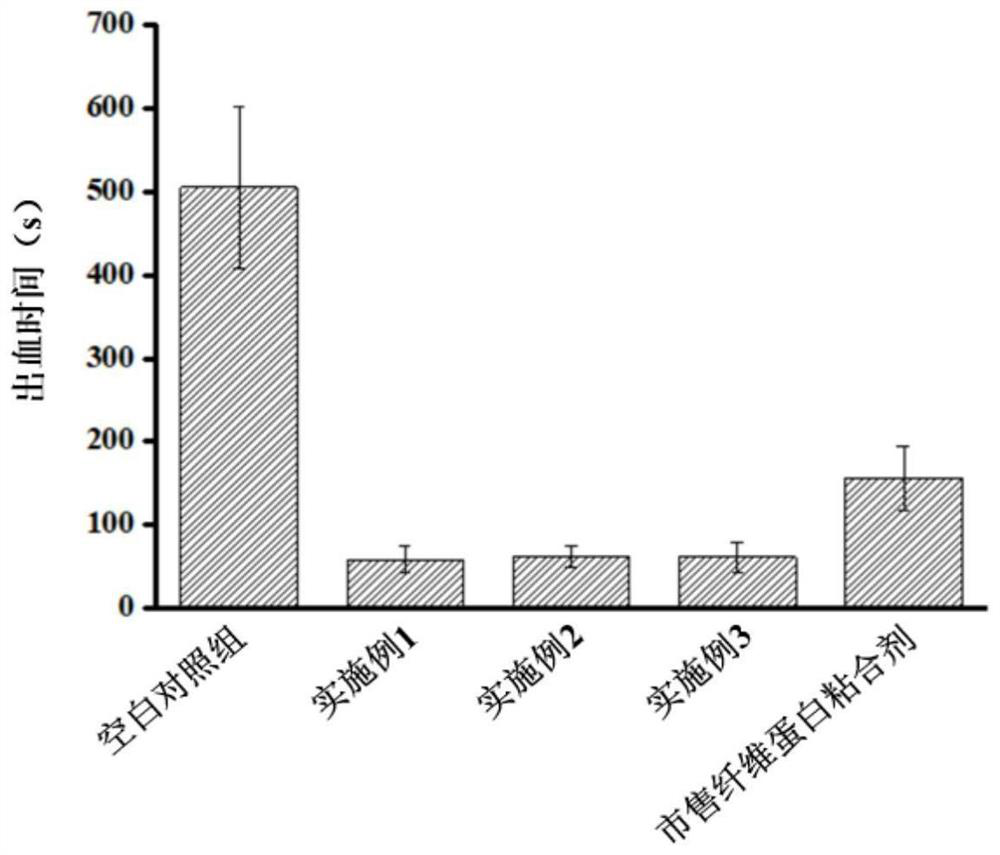
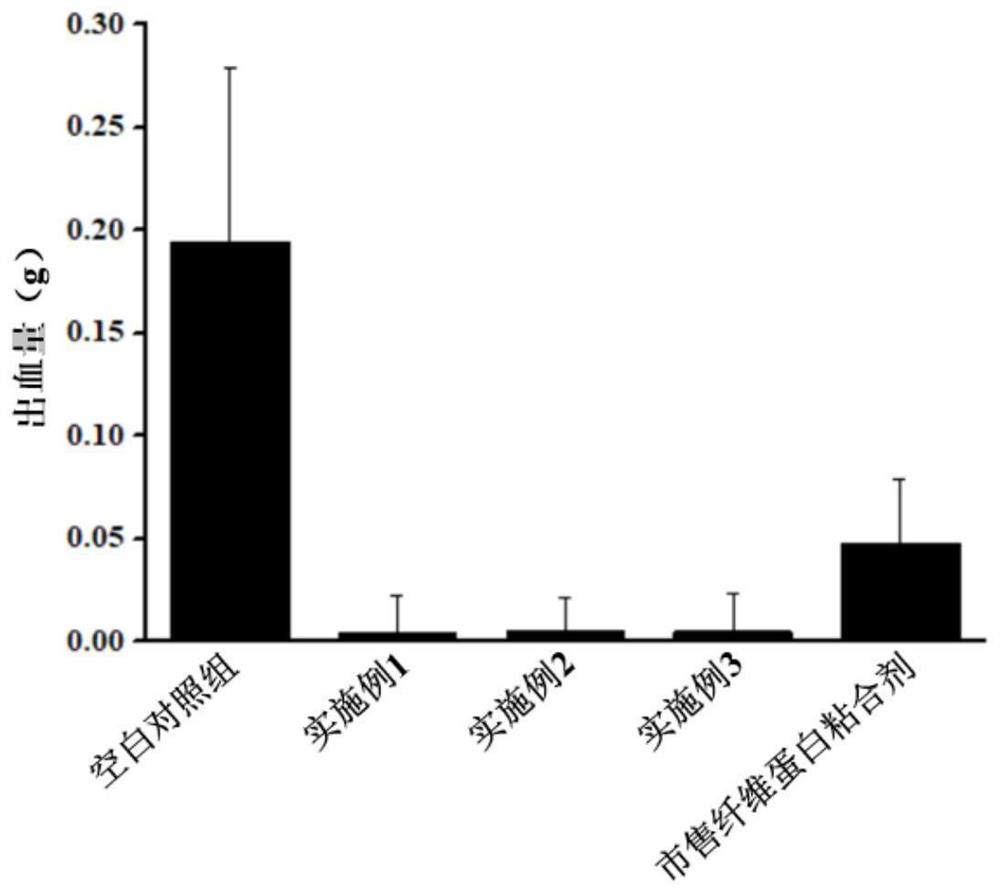
Method for preparing hemostatic material based on air jet pulverization technology
PendingCN113797325ASimple preparation processShort cyclePowder deliveryPeptide/protein ingredientsSucroseFreeze-drying
Owner:FUDAN UNIV
Popular searches
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap