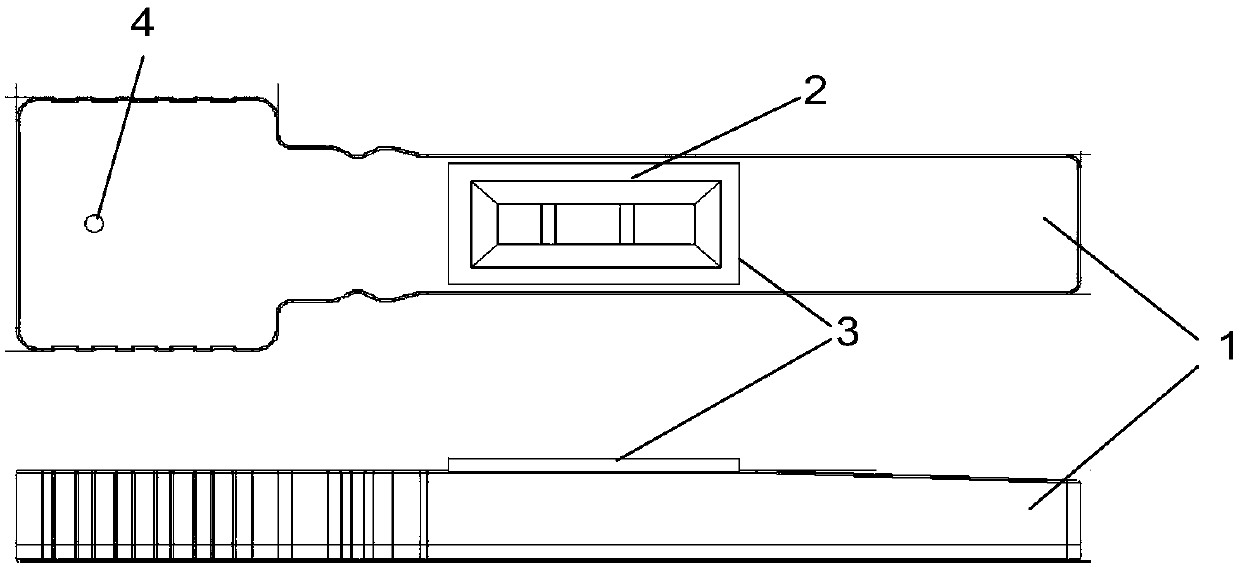
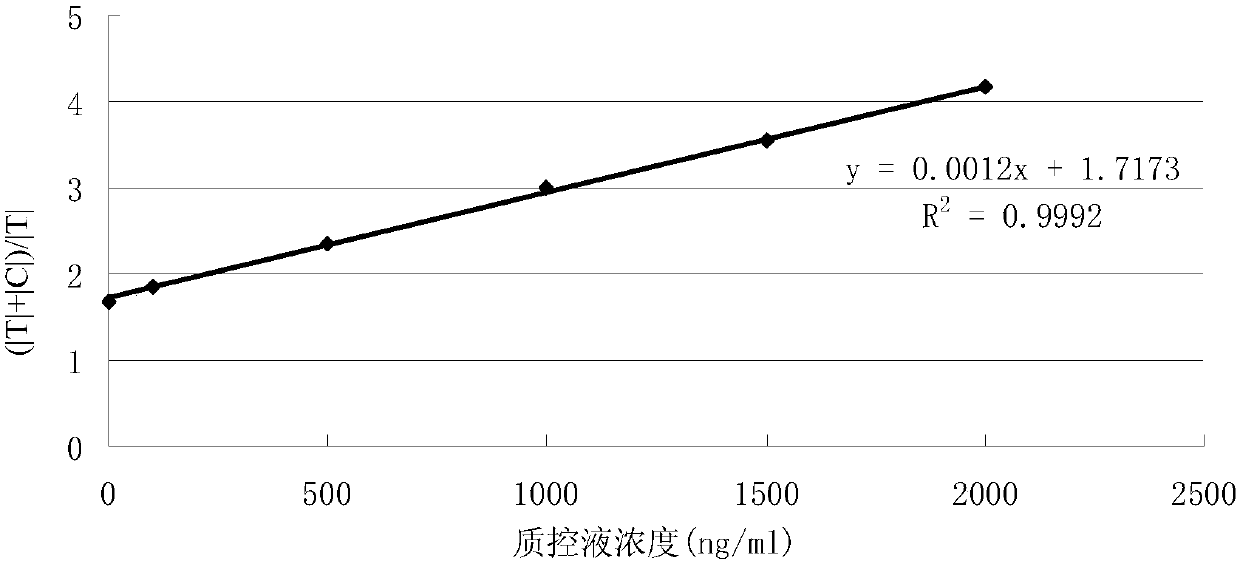
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
7 results about "Linear range" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The linear range is that range of input or output values for which an electronic amplifier produces an output signal that is a direct, linear function of the input signal. When operating in the linear range, no clipping occurs. If an amplifier were perfectly linear, no distortion (harmonic distortion or intermodulation distortion) would occur (although random noise may still be introduced).
Precision improvement calibrating method for current vortex sensor
InactiveCN101793493AHigh precisionAdapt to the actual needs of the projectUsing electrical meansConverting sensor output electrically/magneticallyElectricityMathematical model
The invention discloses a precision improvement calibrating method for a current vortex sensor, which is used for calibrating precision by finding an optimal linear range in which the current vortex sensor can meet requirements so that the current vortex sensor measures at the highest precision in the range meeting the requirements and the established mathematical models are simultaneously minimum. The invention has the advantages of convenient use, high stability and good economical efficiency and furthest utilizes the existing measuring range of the current vortex sensor on the basis of improving the precision of the sensor. A method in the greatest range simplifies the error correction process of the sensor and achieves the purpose of improving the measuring precision by using minimum mathematical models.
Owner:HEFEI UNIV OF TECH
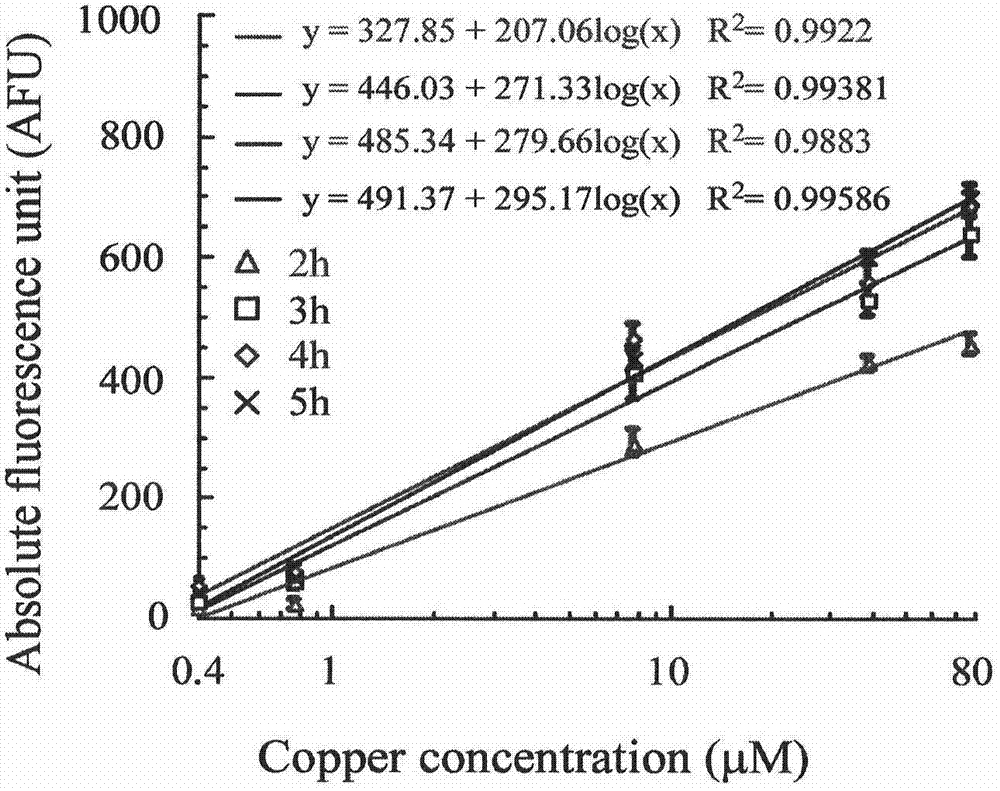
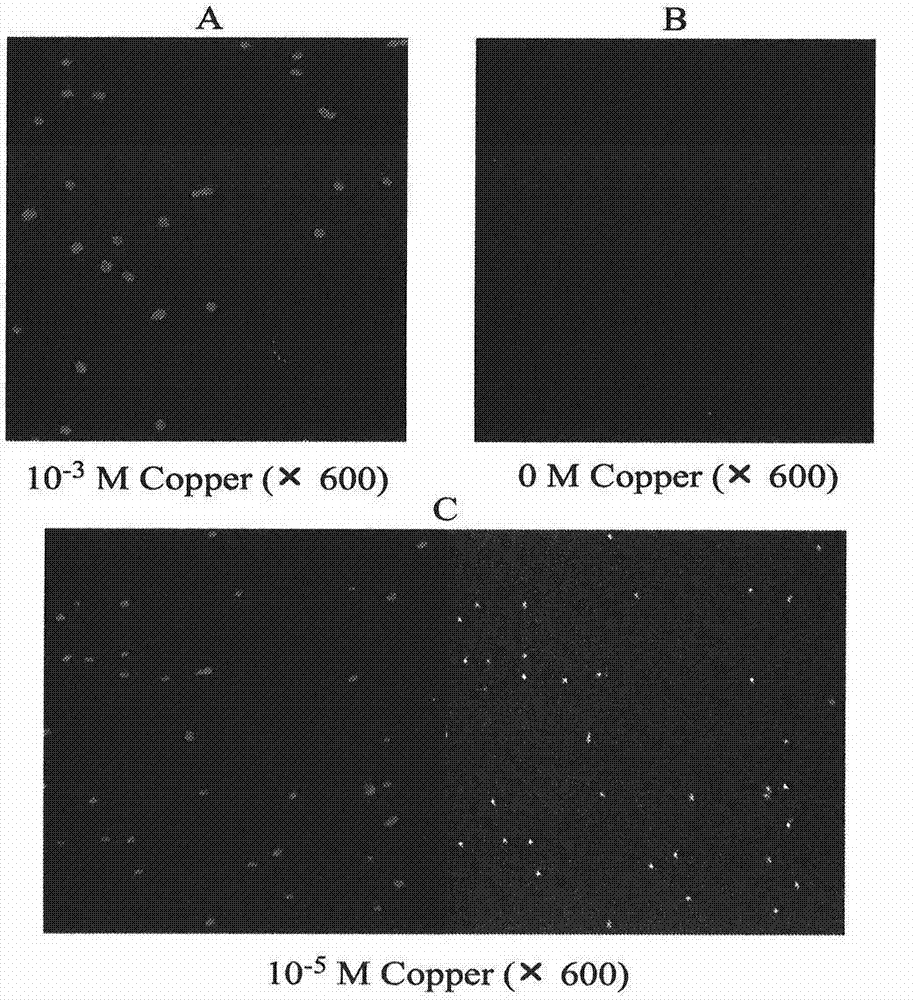
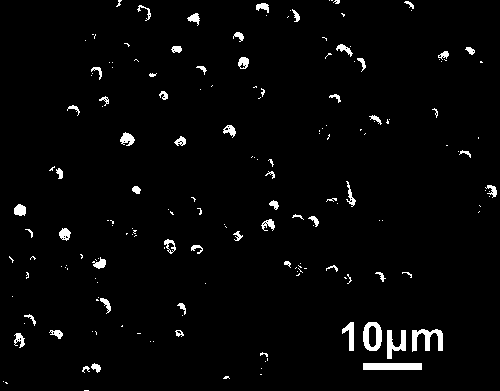
Microbiological method for detection of heavy metal copper in water body
ActiveCN104845998AEasy to detectNo observationBacteriaMicrobiological testing/measurementEscherichia coliContaminated water
Owner:WENZHOU MEDICAL UNIV
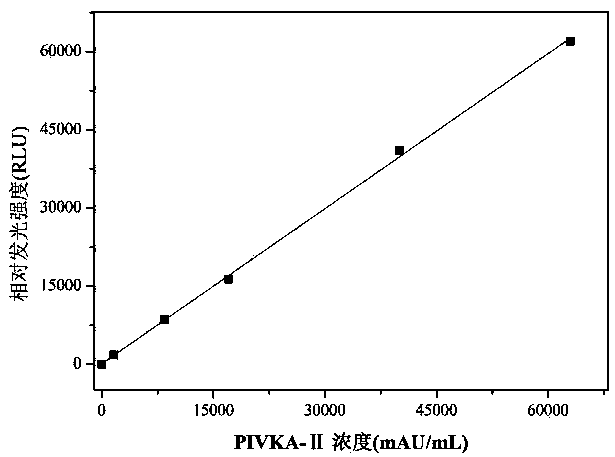
PIVKA-II (protein induced by vitamin K absence-II) magnetic particle chemiluminescence immune assay determination kit and preparation method thereof
PendingCN109425740AHigh sensitivityEasy to operateMaterial analysisBlood plasmaFluorescein isothiocyanate
Owner:江苏麦得科生物科技有限公司
Dry chemical reagent tablet for quantitatively measuring concentration of total cholesterol and preparation method of dry chemical reagent tablet
InactiveCN109916890ANo preparation requiredEasy to operateMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsHemolysisPeroxidase
Owner:吉林省汇酉生物技术股份有限公司
Tyrosine-sensing electrochemical working electrode
InactiveCN107607602AReduce manufacturing costImprove electrocatalytic activityMaterial analysis by electric/magnetic meansTyrosineCopper
Owner:HARBIN UNIV OF SCI & TECH
Test card capable of avoiding influence of wet gas on accuracy in test process and application of test card
InactiveCN107782894AAvoid influenceQuantitative test bias reductionBiological testingHigh humidityThin layer
Owner:BEIJING YICHENG BIOELECTRONICS TECHNOLOGY COMPANY
Method for detecting MFG-E8
InactiveCN107643398AAdequate responseIncreased sensitivityMaterial analysisHorseradish peroxidasePolyclonal antibodies
The invention provides a method for detecting MFG-E8. The method includes 1), preparing detection samples and standard substances; 2), coating and adsorbing anti-human MFG-E8 protein mouse monoclonalantibodies in coated plates; 3), allowing the detection samples and the standard substances to be in contact with the coated anti-human MFG-E8 protein mouse monoclonal antibodies, adding anti-human MFG-E8 protein sheep polyclonal antibodies, anti-sheep IgG rabbit antibodies labeled by horseradish peroxidase and luminescence substrates into the detection samples and the standard samples, allowing the detection samples and the standard substances to be in contact with the anti-human MFG-E8 protein sheep polyclonal antibodies, the anti-sheep IgG rabbit antibodies labeled by the horseradish peroxidase and the luminescence substrates, carrying out light protective reaction, measuring chemiluminescence values and computing the content of the MFG-E8 in the detection samples. The method has the advantages that the method is good in precision, low in detection limit, high in sensitivity and wide in linear range, clinical application requirements can be met, and the like.
Owner:WUHAN CHINESE & WESTERN MEDICINE UNION HOSPITAL
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap