Synthesis method of platy-ZnSe fluorescent nano monocrystal
A fluorescent nano-synthesis method, applied in the field of synthesis of nano-materials, can solve the problems of preparation of flaky ZnSe nano-single crystals, long photodegradation time, and difficulty in washing nano-crystals.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
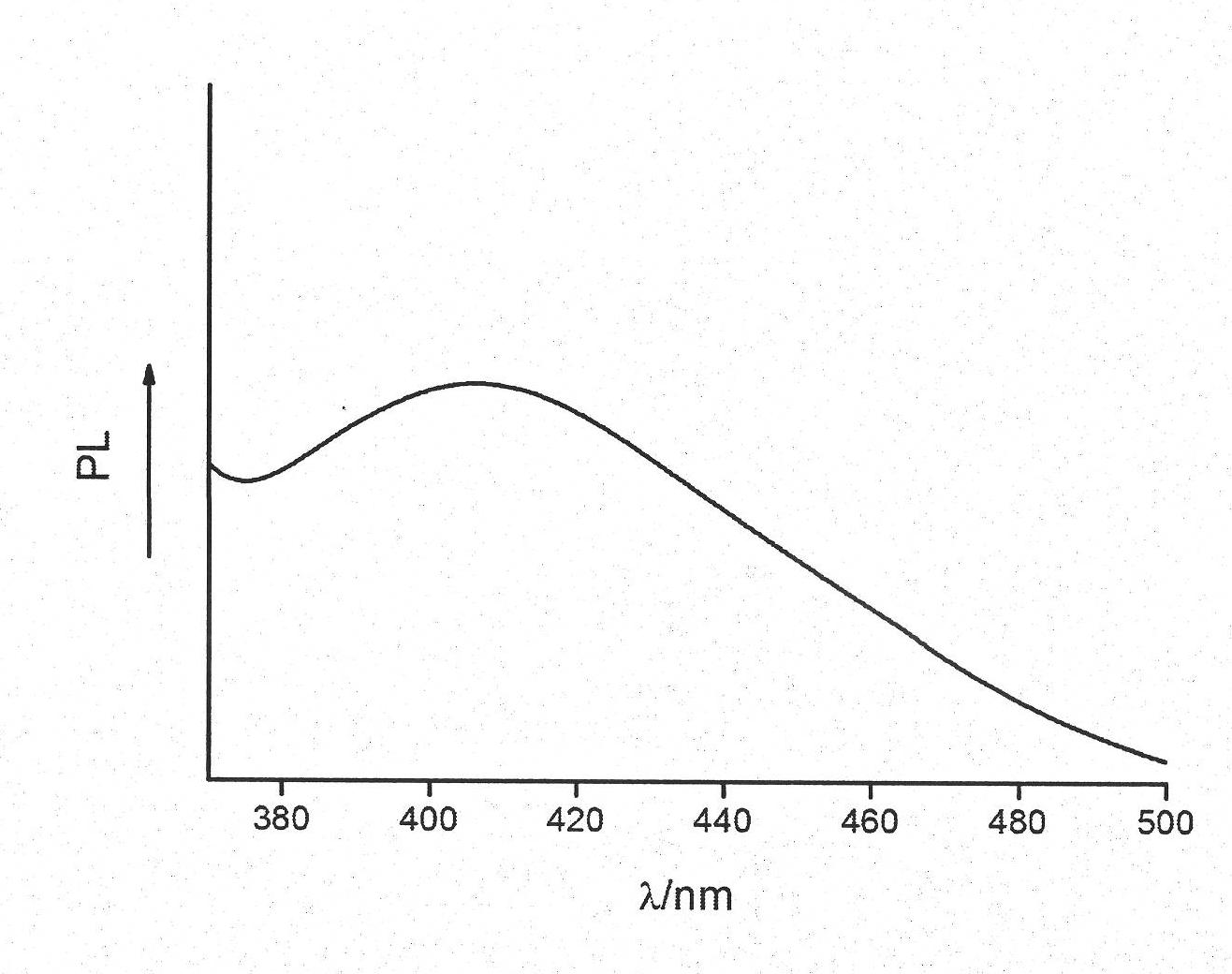
Image
Examples
Embodiment 1
[0047] Synthesis of flaky ZnSe fluorescent nano single crystals:
[0048] 1. First, heat 0.6 mmol of selenium and 1 g of trioctylphosphine in an oil bath at 40° C. under the protection of nitrogen, and stir for one hour to obtain a selenium precursor.
[0049] 2. Stir and mix 2 g of octadecene, 0.085 mmol of zinc stearate, and 0.4 mmol of octadecylamine under the protection of nitrogen to obtain a zinc precursor. The zinc precursor was "activated" by stirring at 50°C for 3 hours.
[0050] 3. Add 8 mmol of octadecyl mercaptan to the zinc precursor under nitrogen protection, and then stir at 50° C. for 1 hour to perform “template” treatment.
[0051] 4. Continue to raise the temperature of the zinc precursor system to 305°C under the protection of nitrogen, and quickly inject the selenium precursor into the zinc precursor while stirring. At this time, the temperature of the reaction system drops to 275°C, and the nanocrystals begin to grow, and the reaction start the timer.
[0
Embodiment 2
[0055] 1. Firstly, 0.6 mmol of selenium and 1 g of trioctylphosphine were heated at 40° C. under the protection of argon and ultrasonicated for half an hour to obtain a selenium precursor.
[0056] 2. Stir and mix 2 g of octadecene, 0.1 mmol of zinc stearate, and 0.8 mmol of octadecylamine under the protection of argon to obtain a zinc precursor. The zinc precursor was "activated" by ultrasonication at 70°C for 1 hour.
[0057] 3. Add 8 mmol of octadecyl mercaptan to the zinc precursor under nitrogen protection, and then stir at 65° C. for 0.5 hour to perform “template” treatment.
[0058] 4. Continue to raise the temperature of the zinc precursor system to 305°C under the protection of argon, and quickly inject the selenium precursor into the zinc precursor while stirring. At this time, the temperature of the reaction system drops to 275°C, and the nanocrystals begin to grow. The reaction starts timing.
[0059] 5. After the nanocrystals start to grow, when the reaction time r
Embodiment 3
[0062] 1. First, 0.6 mmol of selenium and 1 g of trioctylphosphine were heated at 40° C. under nitrogen protection and ultrasonicated for half an hour to obtain a selenium precursor.
[0063] 2. Fully grind the zinc precursor composed of 2 g of octadecene, 0.085 mmol of zinc stearate, and 0.8 mmol of hexadecylamine at 25° C. for 3 hours to realize its “activation” treatment.
[0064] 3. Add 4 mmol of octadecyl mercaptan to the zinc precursor under nitrogen protection, and then stir at 70° C. for 1 hour to perform “template” treatment.
[0065] 4. Under the protection of nitrogen, raise the temperature of the zinc precursor system to 315°C, quickly inject the selenium precursor into the zinc precursor while stirring, at this time, the temperature of the reaction system drops to 290°C, the nanocrystals begin to grow, and the reaction start the timer.
[0066] 5. After the nanocrystals start to grow, when the reaction time reaches 1 hour and 30 minutes, stop heating and end the rea
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap