Method for controlling surface potentials of graphene-based material
A surface potential, graphene-based technology, applied in chemical instruments and methods, inorganic chemistry, non-metallic elements, etc., can solve cumbersome problems and achieve high reaction temperature, strong reduction, and strong oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
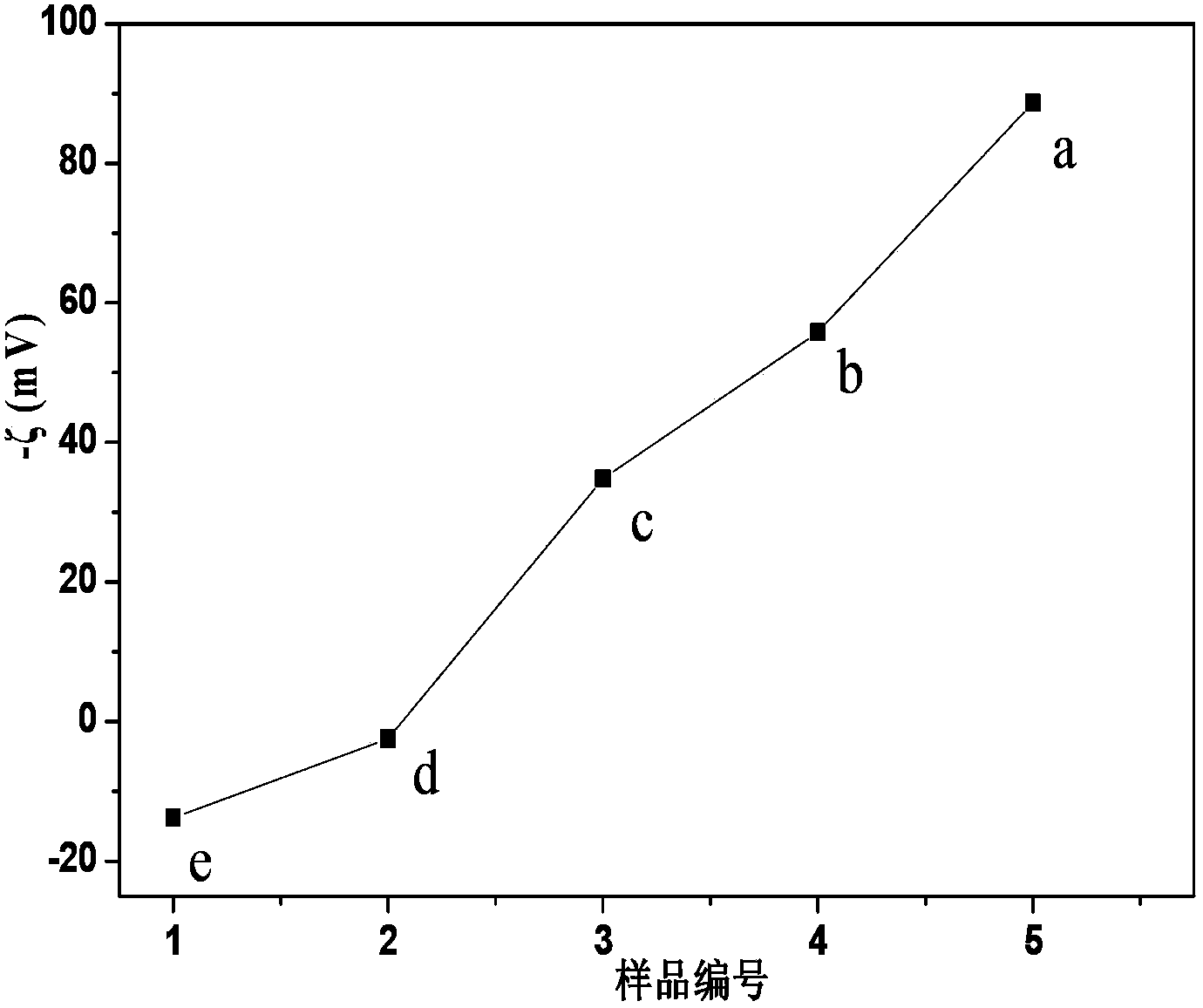
[0027] Press the graphite oxide powder into 0.5mg ml -1Disperse in ultrapure water, and ultrasonically treat in a water bath for 2 hours to obtain a graphene oxide dispersion. Add a certain amount of 80% hydrazine hydrate (mass ratio of graphite oxide to graphite is 1:1) into the above dispersion liquid, ultrasonically disperse in a water bath for 5 minutes, and mix well. The above mixed solution was put into a round bottom flask, kept at 35° C. in a water bath, and refluxed for 1 h under magnetic stirring. The product was separated by a high-speed centrifuge, washed several times with ultrapure water, centrifuged, and dried at 55°C. Disperse the resulting solid powder in ultrapure water at a concentration of 0.05 mg ml -1 , ultrasonically disperse in a water bath for 5 minutes, and adjust the pH to 7 with 0.1M hydrochloric acid and 0.1M ammonia water. The zeta potential test result is -55.8mV ( figure 1 in b). This graphene can be used to adsorb heavy metal cations or to ma
Embodiment 2
[0029] Press the graphite oxide powder into 0.5mg ml -1 Disperse in ultrapure water, and ultrasonically treat in a water bath for 2 hours to obtain a graphene oxide dispersion. Add a certain amount of 80% hydrazine hydrate (mass ratio of graphite oxide to graphite is 1:1) into the above dispersion liquid, ultrasonically disperse in a water bath for 5 minutes, and mix well. The above mixed solution was put into a round bottom flask, kept at 65°C in a water bath, and refluxed for 12 hours under magnetic stirring. The product was separated by a high-speed centrifuge, washed several times with ultrapure water, centrifuged, and dried at 55°C. Disperse the resulting solid powder in ultrapure water at a concentration of 0.05 mg ml -1 , ultrasonically disperse in a water bath for 5 minutes, and adjust the pH to 7 with 0.1M hydrochloric acid and 0.1M ammonia water. The zeta potential test result is -34.8mV ( figure 1 in c). This graphene can be used to adsorb cations with a small mas
Embodiment 3
[0031] Press the graphite oxide powder into 1mg ml -1 Disperse in a solvent, and ultrasonically treat in a water bath for 2 hours to obtain a graphene oxide dispersion. Put a certain amount of 1mg ml -1 The p-phenylenediamine ethanol solution and the above dispersion liquid were mixed in equal volume, ultrasonically dispersed in a water bath for 30 minutes, and mixed evenly. The above mixed solution was put into a round bottom flask, kept at 45°C in a water bath, and refluxed for 12 hours under magnetic stirring. The product was separated with a high-speed centrifuge, washed with washing solution (a mixed solution of ultrapure water and ethanol at a volume ratio of 1:1), centrifuged, and dried at 55°C. Disperse the resulting solid powder in ultrapure water at a concentration of 0.05 mg ml -1 , ultrasonically disperse in a water bath for 30 minutes, and adjust the pH to 3.5 with 0.1M hydrochloric acid and 0.1M ammonia water. The zeta potential test result is 2.5mV ( figure 1
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap