Synthetic method of water soluble ultraviolet light absorber BP-9
A technology of BP-9, synthesis method, applied in the preparation of sulfonate, organic chemistry and other directions, can solve the problems of complex sulfonation process, acid waste water pollution, poor sulfonation selectivity, etc., and achieve high yield and purity, corrosiveness Small, stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
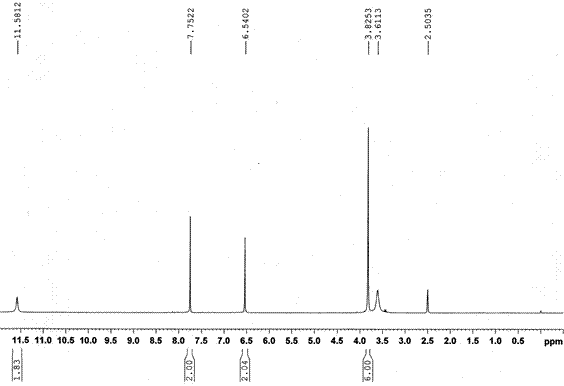
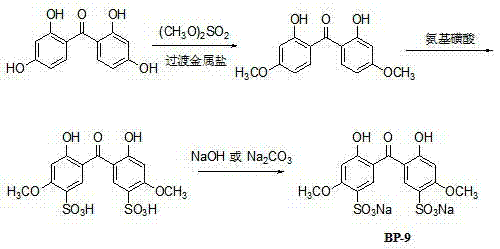
[0026] ① Methylation reaction: Put 123kg of 2,2',4,4'-tetrahydroxybenzophenone, 2.95kg of zinc chloride and a mixture of 295L dichloroethane + 295L absolute ethanol into the reaction kettle Solvent 590L, heat and stir to dissolve, heat up to 45~50°C, add 148kg of dimethyl sulfate dropwise, 2h is completed, keep warm for 6h; cool to room temperature, add 148L of water, stir to dissolve, then stand to separate layers, and separate the organic phase , the water phase was extracted twice with dichloroethane, each time 50L, the organic phase was combined, the solvent was recovered by distillation, and cooled to crystallize to obtain 2,2'-dihydroxy-4,4'dimethoxybenzophenone About 130kg, molar yield 95% (based on 2,2',4,4'-tetrahydroxybenzophenone);
[0027] ②Sulfonation reaction: Put 130kg of the methylated product 2,2'-dihydroxy-4,4'dimethoxybenzophenone and 610 L of dichloroethane into the reactor, heat and stir to dissolve , heated up to 60~65°C, put 112kg of sulfamic acid in 3 ...
Embodiment 2
[0031] ① Methylation reaction: Put 123kg of 2,2',4,4'-tetrahydroxybenzophenone, 2.46kg of copper chloride and a mixture of 276L dichloroethane + 276L absolute ethanol into the reaction kettle Solvent 552L, heat and stir to dissolve, heat up to 45~50°C, add 148kg of dimethyl sulfate dropwise, after 2h dropwise addition, keep warm for 6h reaction; cool to room temperature, add 148L of water, stir to dissolve, then stand for stratification, and separate the organic phase , the aqueous phase was extracted twice with dichloroethane, 50 L each time, the organic phases extracted twice were combined, the solvent was recovered by distillation, and crystallized by cooling to obtain 2,2'-dihydroxy-4,4'dimethoxydi Benzophenone 124kg;
[0032] ②Sulfonation reaction: Put 124kg of the methylated product 2,2'-dihydroxy-4,4'dimethoxybenzophenone and 596L of chloroform into the reactor, heat and stir to dissolve, and heat up To 60~65°C, add 99kg of sulfamic acid in 3 times, keep it warm for 5...
Embodiment 3
[0035] ①Methylation reaction: Put 123kg of 2,2',4,4'-tetrahydroxybenzophenone, 3.1kg of nickel chloride and a mixture of 307L dichloroethane + 307L absolute ethanol into the reaction kettle Solvent 614L, heat and stir to dissolve, heat up to 45~50°C, add 184.5kg of dimethyl sulfate dropwise, dropwise add 2h, keep warm for 6h; cool to room temperature, add 185L of water and stir to dissolve, then stand for stratification, separate organic phase, the aqueous phase was extracted twice with dichloroethane, 55 L each time, the organic phases were combined twice, the solvent was recovered by distillation, and crystallized by cooling to obtain 2,2'-dihydroxy-4,4'dimethoxydi Benzophenone 127kg;
[0036] ②Sulfonation reaction: Put 127kg of the methylated product 2,2'-dihydroxy-4,4'dimethoxybenzophenone and 610 L of dichloroethylene into the reactor, heat and stir to dissolve, Raise the temperature to 60~65°C, add 112 kg of sulfamic acid in 3 times, keep the temperature for 5 hours, co...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap