Method for removing heavy metals in water body
A technology for heavy metals and water bodies, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems that the decomposition products cannot be treated, the permanganate oxidation ability is limited, and cannot be oxidized and removed, achieving remarkable results Environmental benefits, no secondary pollution, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
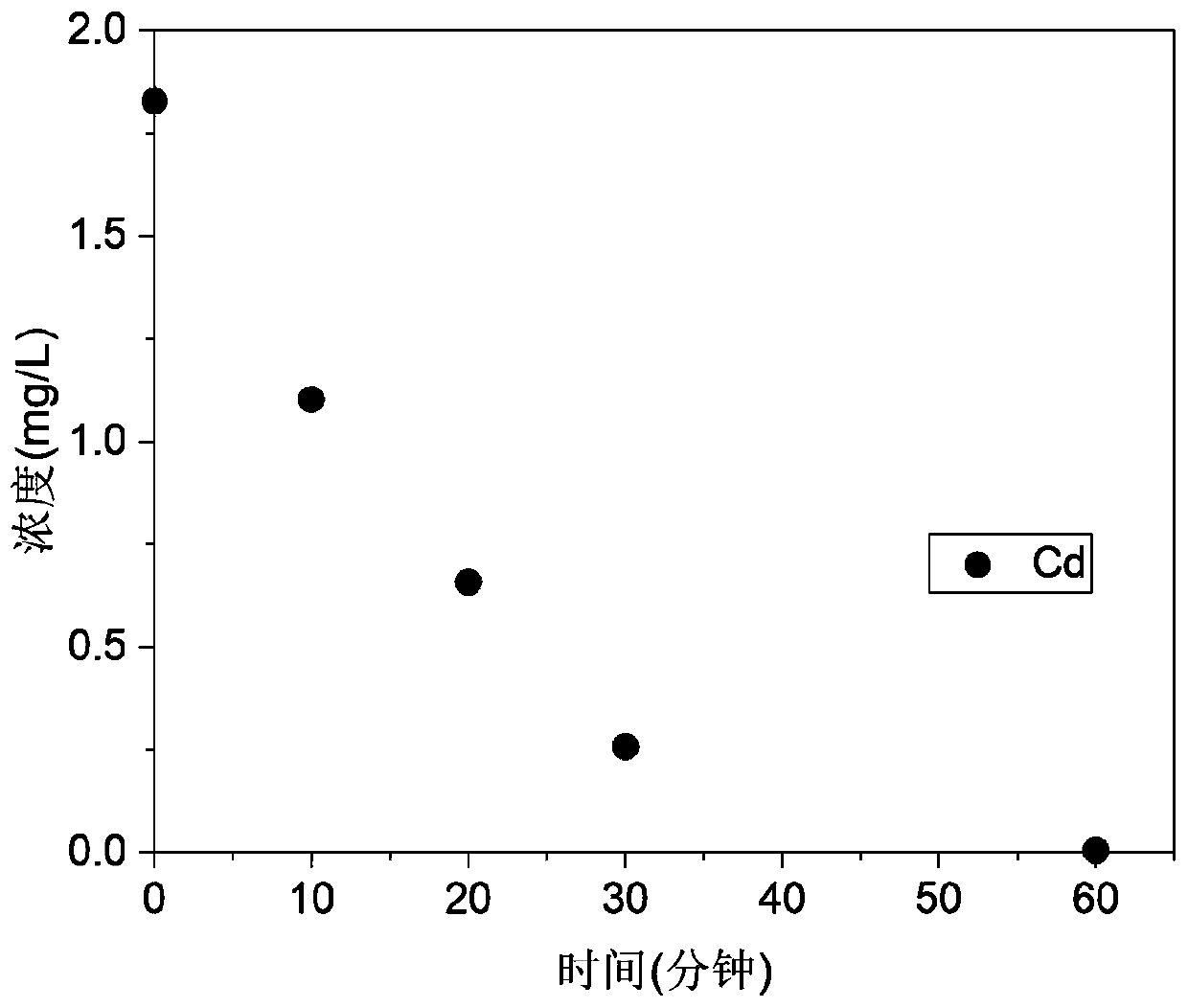
[0042] Preparation of water body to be treated: Add 1mL of cadmium nitrate (Cd(NO 3 ) 2 ), so that the concentration of Cd(II) in the system is 20 μmol / L.
[0043] Inorganic state heavy metal Cd removal treatment: use dilute nitric acid and sodium hydroxide solution to adjust the initial pH value of the water to be treated to 6, then add potassium permanganate solution to the water body, and carry out the reaction of removing inorganic heavy metals under light irradiation; The concentration of potassium manganate is 100μmol / L; the light wavelength is 254nm, and the light intensity is 2.07mW·cm -2 ; The light irradiation method is immersion; the heavy metal removal reaction is carried out at a room temperature of 25°C under stirring conditions, and the oxidation-adsorption reaction time is 60min.
[0044] After the water body was diluted with 2% dilute nitric acid solution, an inductively coupled plasma mass spectrometer ICP-MS (model NexION 350D) was used to measure the con
Embodiment 2
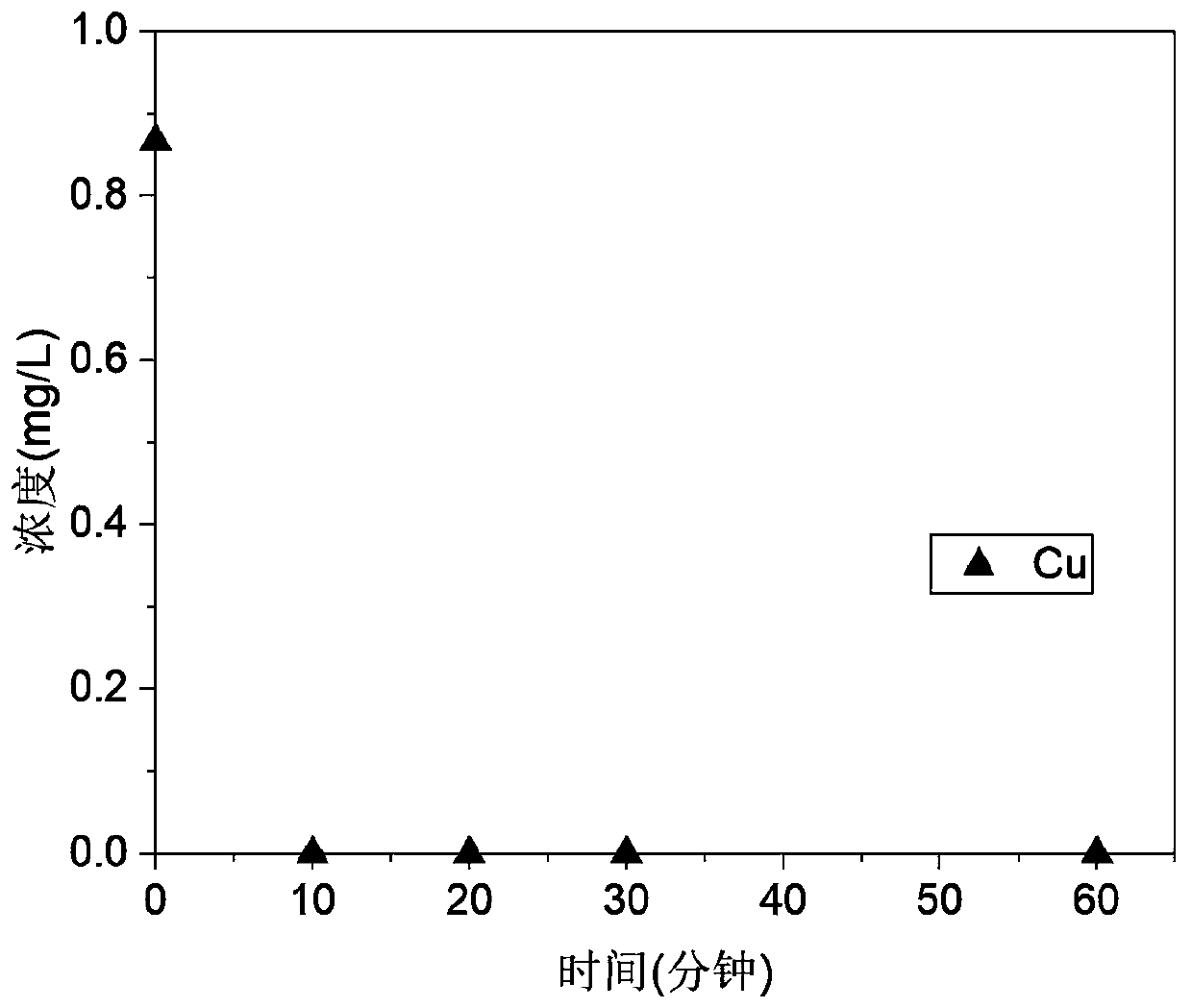
[0052] Preparation of water body to be treated: Add 1 mL of copper nitrate (Cu(NO 3 ) 2 ), so that the concentration of Cu(II) in the system is 20 μmol / L.
[0053] Inorganic state heavy metal Cu removal treatment: use dilute nitric acid and sodium hydroxide solution to adjust the initial pH value of the water to be treated to 6, then add potassium permanganate solution to the water body, and carry out the reaction of removing inorganic heavy metals under light irradiation; The concentration of potassium manganate is 100μmol / L; the light wavelength is 254nm, and the light intensity is 2.07mW·cm -2 ; The light irradiation method is immersion; the heavy metal removal reaction is carried out at a room temperature of 25°C under stirring conditions, and the oxidation-adsorption reaction time is 60min.
[0054] After the water body was diluted with 2% dilute nitric acid solution, an inductively coupled plasma mass spectrometer ICP-MS (model NexION 350D) was used to measure the con
Embodiment 3
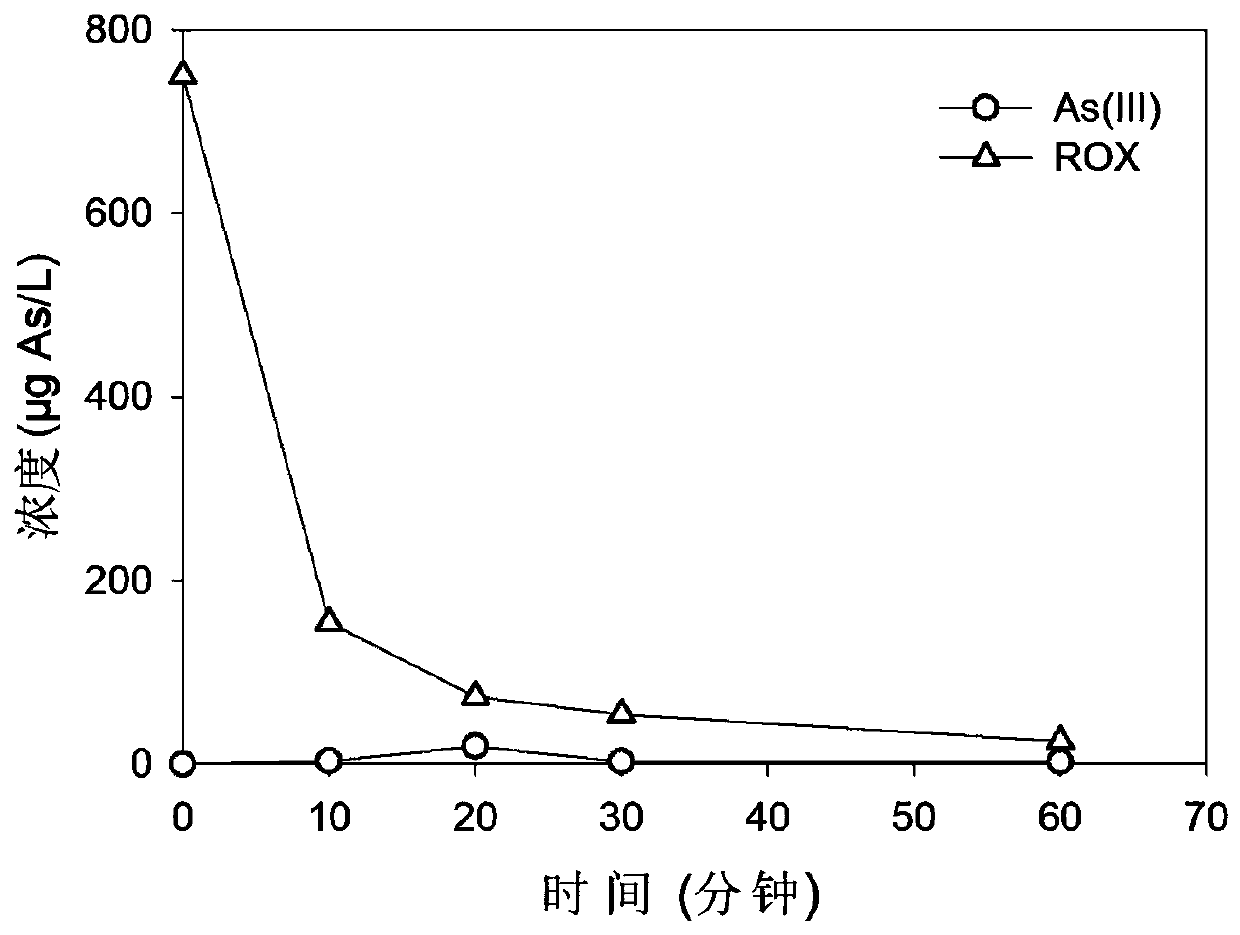
[0062] Preparation of water body to be treated: add ROX to 700mL ultrapure water, and the concentration of ROX is 10μmol / L.
[0063] ROX removal treatment of organic heavy metal substances: use dilute hydrochloric acid and sodium hydroxide solution to adjust the initial pH value to 7, then add potassium permanganate solution to the water body, and carry out the reaction of removing organic heavy metals under light irradiation; among them, high manganese The potassium acid concentration is 100μmol / L; the light wavelength is 254nm, and the light intensity is 2.07mW·cm -2 ; The light irradiation method is immersion; the removal of organic heavy metals is carried out at a room temperature of 25 ° C under stirring conditions, and the oxidation-adsorption reaction time is 60 minutes.
[0064] Adopt liquid chromatography coupled with inductively coupled plasma mass spectrometer LC-ICP-MS, measure the concentration content of arsenic element in water body in the oxidation-adsorption
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap