Method for increasing etec cs6 antigen presentation on cell surface and products obtainable thereof
a technology of cs6 and antigen, which is applied in the field of methods useful in the preparation of etec cs6 antigen, can solve the problems of reducing the immunological activity of cs6 protein in the immune response of e. coli /i>, and achieving the effect of increasing the amount of cs6 antigen on the cell surfa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of CS6 in E. Coli C600-CS6
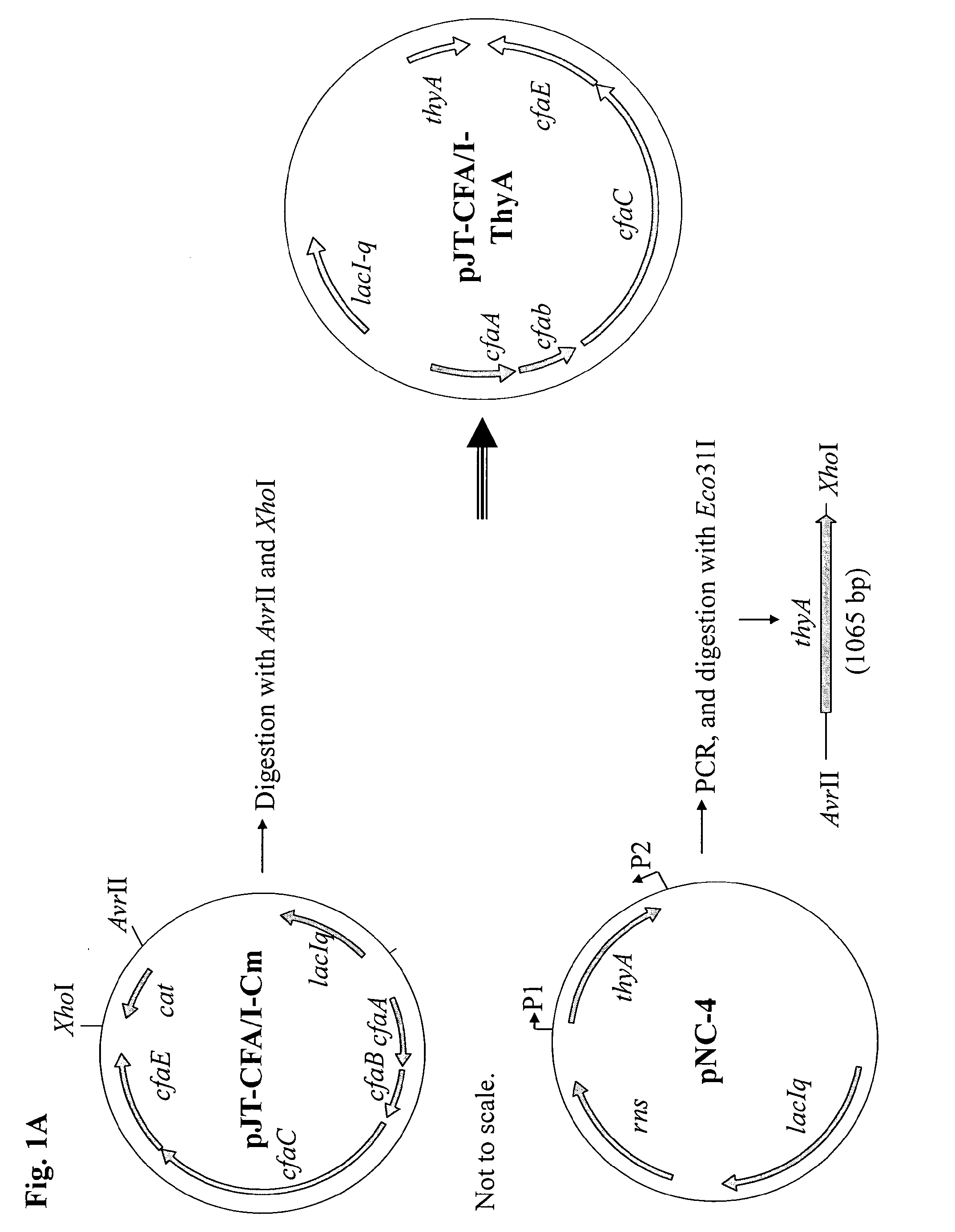
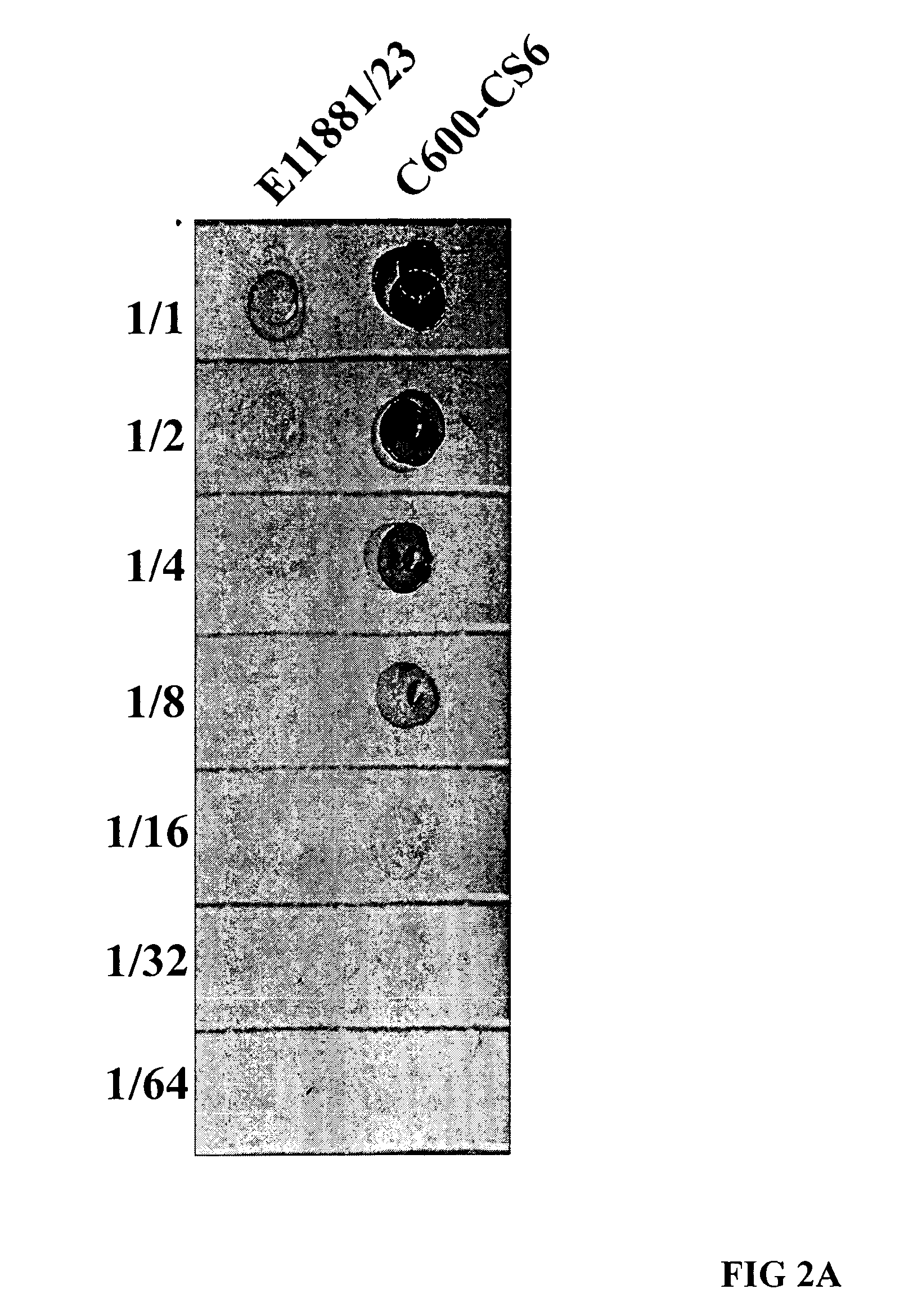
[0044]A DNA fragment carrying the structural genes (cssA, cssB, cssC, cssD) for CS6, prepared from a wild-type ETEC strain with surface expression of CS6, was amplified by PCR and cloned to construct the expression vector pJT-CS6-ThyA, as depicted in FIGS. 1A and 1B. This plasmid was then electroporated into the thymine dependent, non-toxigenic E. coli C600-ΔthyA strain, and CS6 surface expression was induced by addition of IPTG to the growth medium, as shown in an immuno-dot blot assay (FIG. 2A). No CS6 expression was observed in the absence of the inducer (data not shown). When examining the expression of CS6 by the recombinant C600-CS6 strain using the dot blot assay, we found that this strain expressed at least 8-fold higher levels of CS6 compared to the CS6 reference strain E11881 / 23, which had previously been used as CS4+CS6 vaccine strain in the CF-CTB-ETEC vaccine (FIG. 2A). Likewise, also when specifically determining the surface expressi
example 2
Inactivation of Bacteria Without Destroying the CS6 Antigen Properties
[0045]With the aim to kill the CS6 expressing bacteria while preserving the CS6 antigen properties on their surface, the effects of formaldehyde and phenol were compared. Preliminary studies showed that treating the bacteria with 0.3% or 0.6% formaldehyde, while safely killing the bacteria, resulted in a complete loss of detectable CS6 antigen (data not shown). In contrast, treating the bacteria with 0.5% phenol not only killed the tested bacteria, but also preserved the CS6 antigen (data not shown); a lower tested concentration of phenol, 0.25%, on the other hand did not result in complete killing of the bacteria. These results indicated that phenol treatment could be useful for inactivating the bacteria while preserving the CS6 antigen. To work out an optimal inactivation method, different concentrations of phenol were therefore tested for inactivation of both the recombinant C600-CS6 strain and for comparison anot
example 3
Immunogenicity of Phenol-Killed C600-CS6 in Mice
[0046]In a first test of the immunogenicity of the recombinant strain C600-CS6 we immunized groups of both Balb / C and C57 Bl / 6 mice with the same number of phenol-killed bacteria, and the serum antibody responses to CS6, as measured by ELISA, were compared. Although significant anti-CS6 responses were induced in both types of mice, the antibody responses in C57 Bl / 6 mice were substantially higher than in the Balb / C mice (data not shown). We therefore used C57 Bl / 6 mice for the further oral immunization studies in which the immunogenicity of orally administered phenol-killed vaccine preparation of C600-CS6 and the reference strain E11881 / 23 were compared. The results showed that all immunized mice responded with production of serum IgG+IgM antibodies against CS6, and that the titers of antibodies against CS6 on average were more than 60-fold higher in mice immunized with C600-CS6 bacteria than those in mice immunized with the r
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap