Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
8 results about "Mammal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mammals (from Latin mamma "breast") are vertebrate animals constituting the class Mammalia (/məˈmeɪliə/), and characterized by the presence of mammary glands which in females (and sometimes males) produce milk for feeding (nursing) their young, a neocortex (a region of the brain), fur or hair, and three middle ear bones. These characteristics distinguish them from reptiles and birds, from which they diverged in the late Triassic, 201–227 million years ago. There are around 5,450 species of mammals. The largest orders are the rodents, bats and Soricomorpha (shrews and others). The next three are the Primates (apes, monkeys, and others), the Cetartiodactyla (cetaceans and even-toed ungulates), and the Carnivora (cats, dogs, seals, and others).
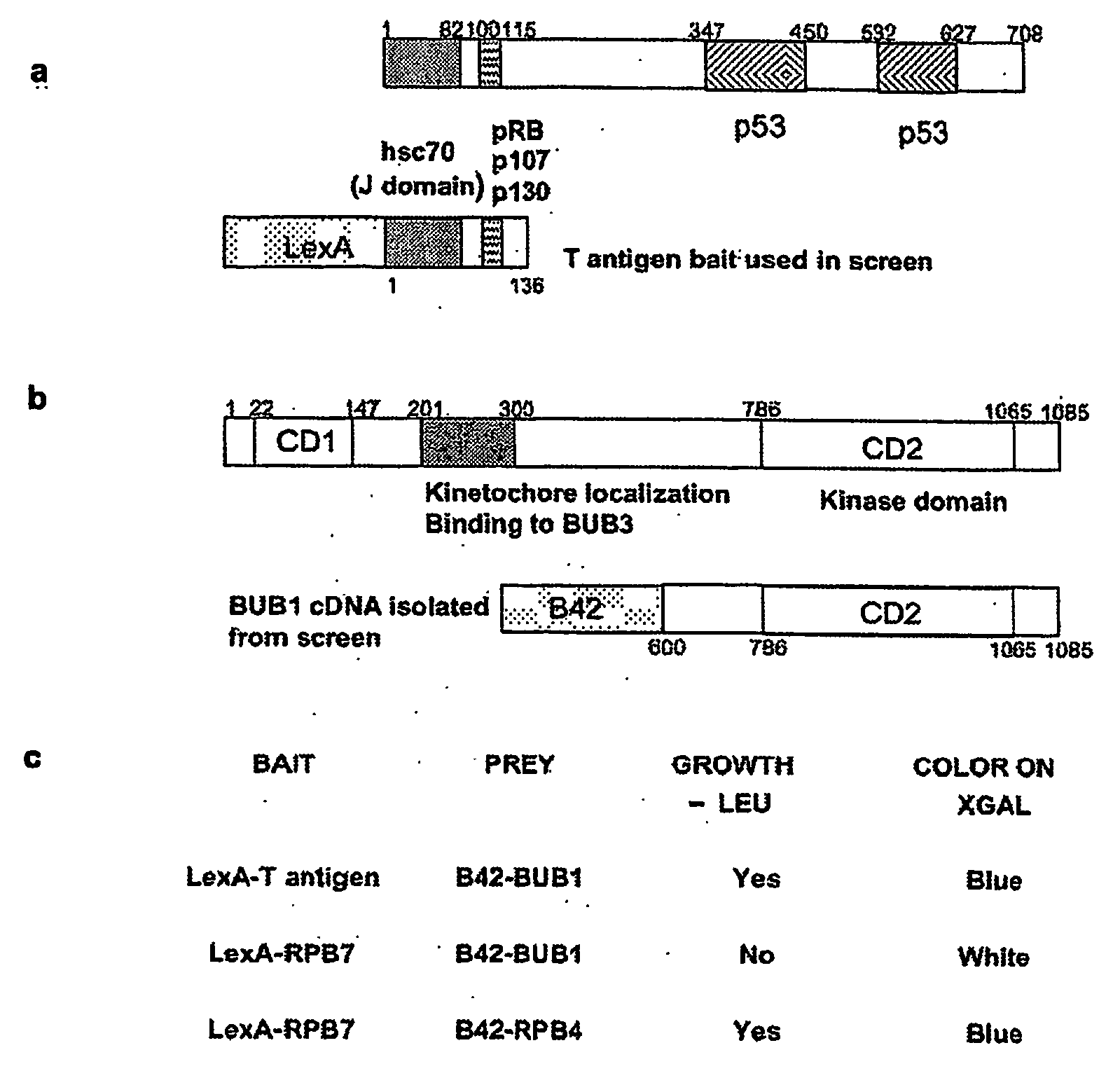
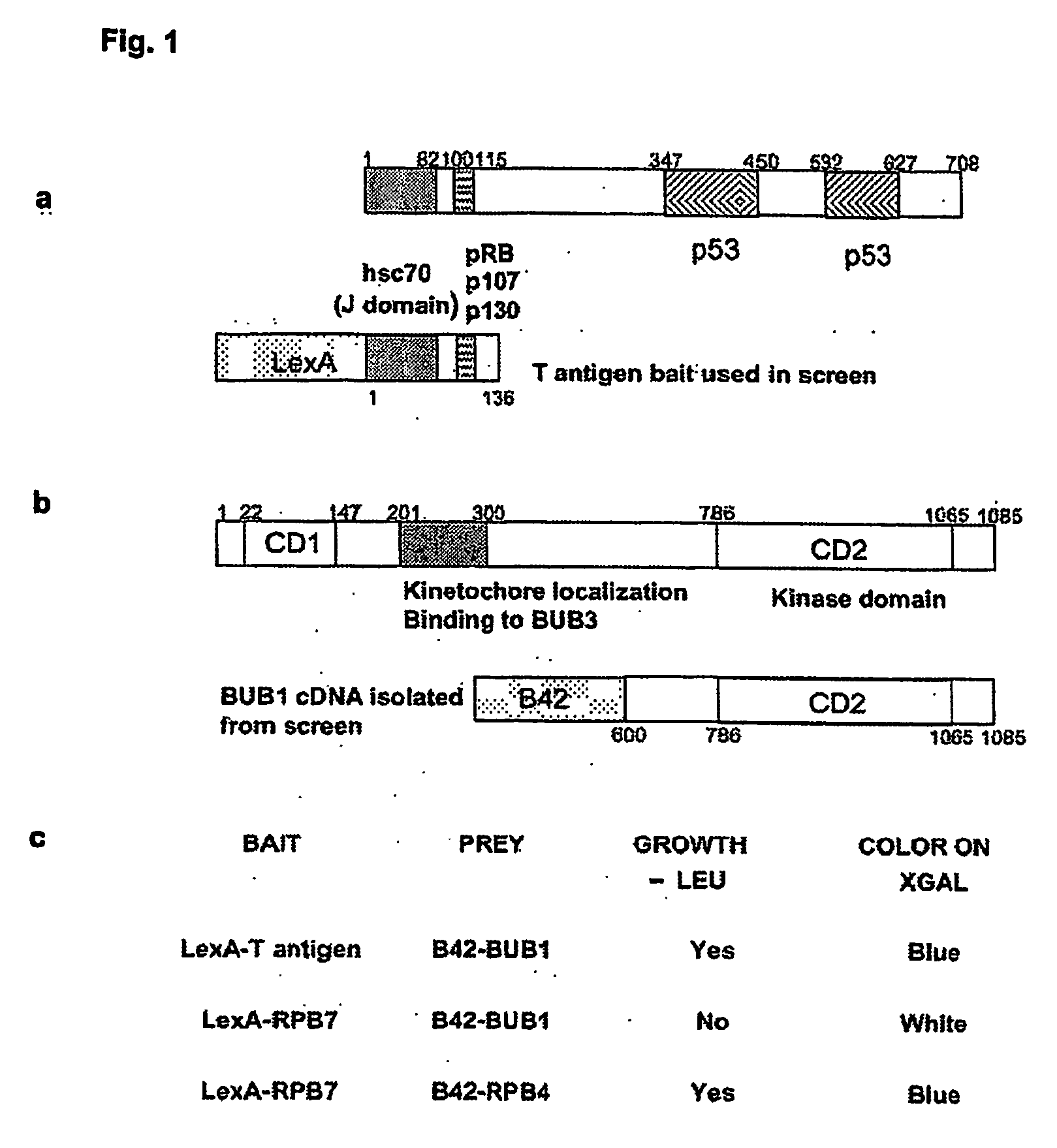
Non-mammalian GnRH analogs and uses thereof in the immune system
InactiveUS20050043245A1Effective supervisionHigh affinityPeptide/protein ingredientsLuteinising hormone-releasing hormoneDiseaseD-Arginine
Specially designed non-mammalian GnRH, its analogs, or biometics resistant to degradation by peptidase, are disclosed. The GnRH analogs are further defined as analogs of GnRH II or salmon GnRH. These non-mammalian analogs incorporate D-arginine, D-leucine, D-tBu-Serine, D-Trp or other active D amino acids at position 6 and ethylamide, aza-Gly-amide or other Gly amide at position 10. The D-Arg (6)—GnRH II-ethylamide, D-Arg (6)—GnRH II-aza-Gly (10)-amide, the D-Arg (6)—salmon GnRH ethylamide, and D-Arg (6)—salmon GnRH-aza-Gly (10)-amide analogs are also provided, and demonstrate preferential binding to immune system non-mammalian GnRH receptors. These non-mammalian GnRH or its analogs, or long-acting preparation, biometics or their antibodies may be used in pharmaceutical preparation, and specifically in treatment of various immune system disorders. The non-mammalian GnRH or its analogs are also provided in pharmaceutical preparations that may be used clinically for treating immune system disorders when used in very low doses and administered in pulsatile fashion. The aza-Gly (10) amide non-mammalian analogs are yet other embodiments of the non-mammalian GnRH or its analogs provided as a part of the invention. The use of agents that regulate the production or antibodies or In addition, the detection of non-mammalian GnRH or GnRH II or the non-mammalian GnRH receptors may be used as a diagnostic tool.
Owner:SILER KHODR THERESA
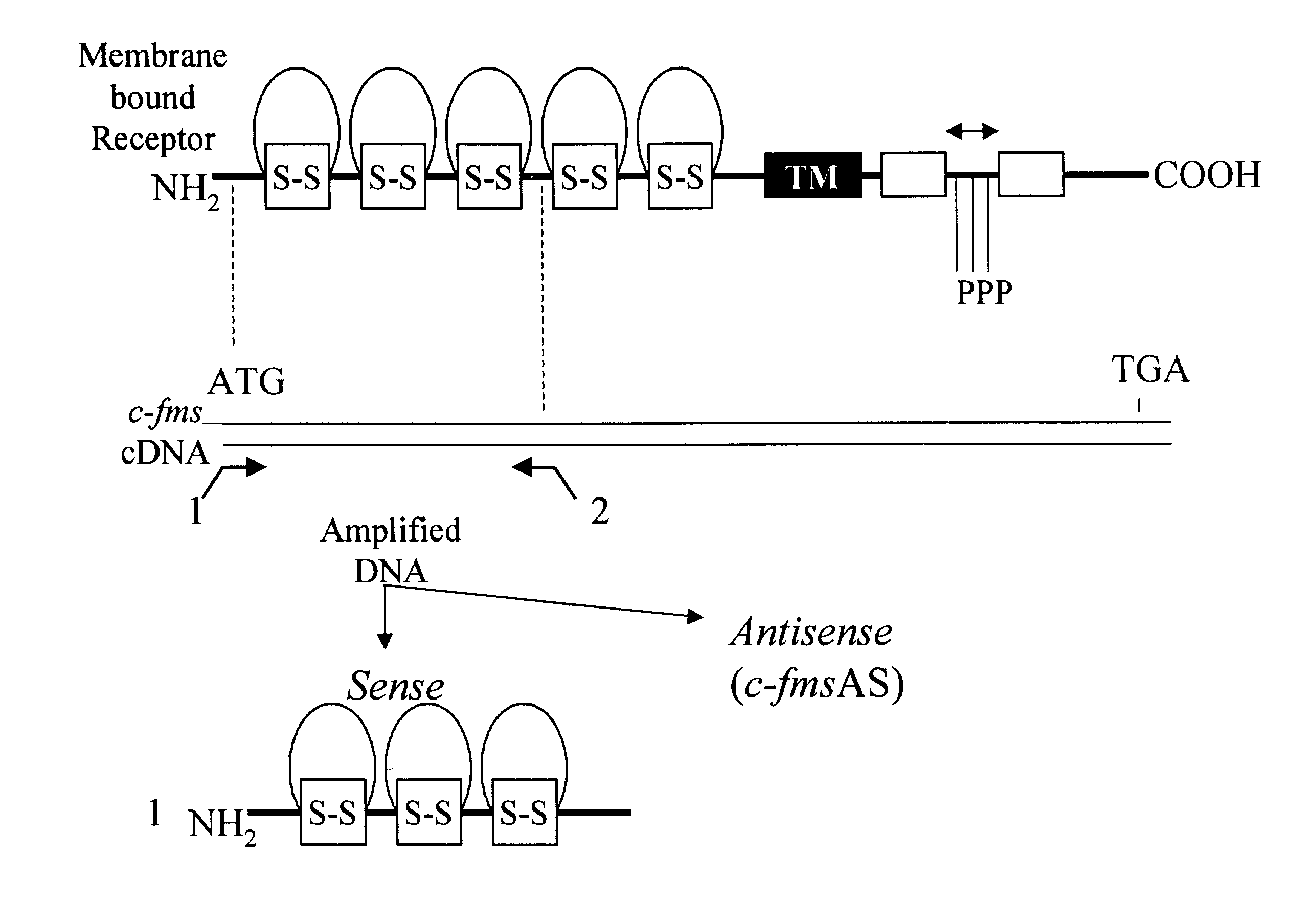
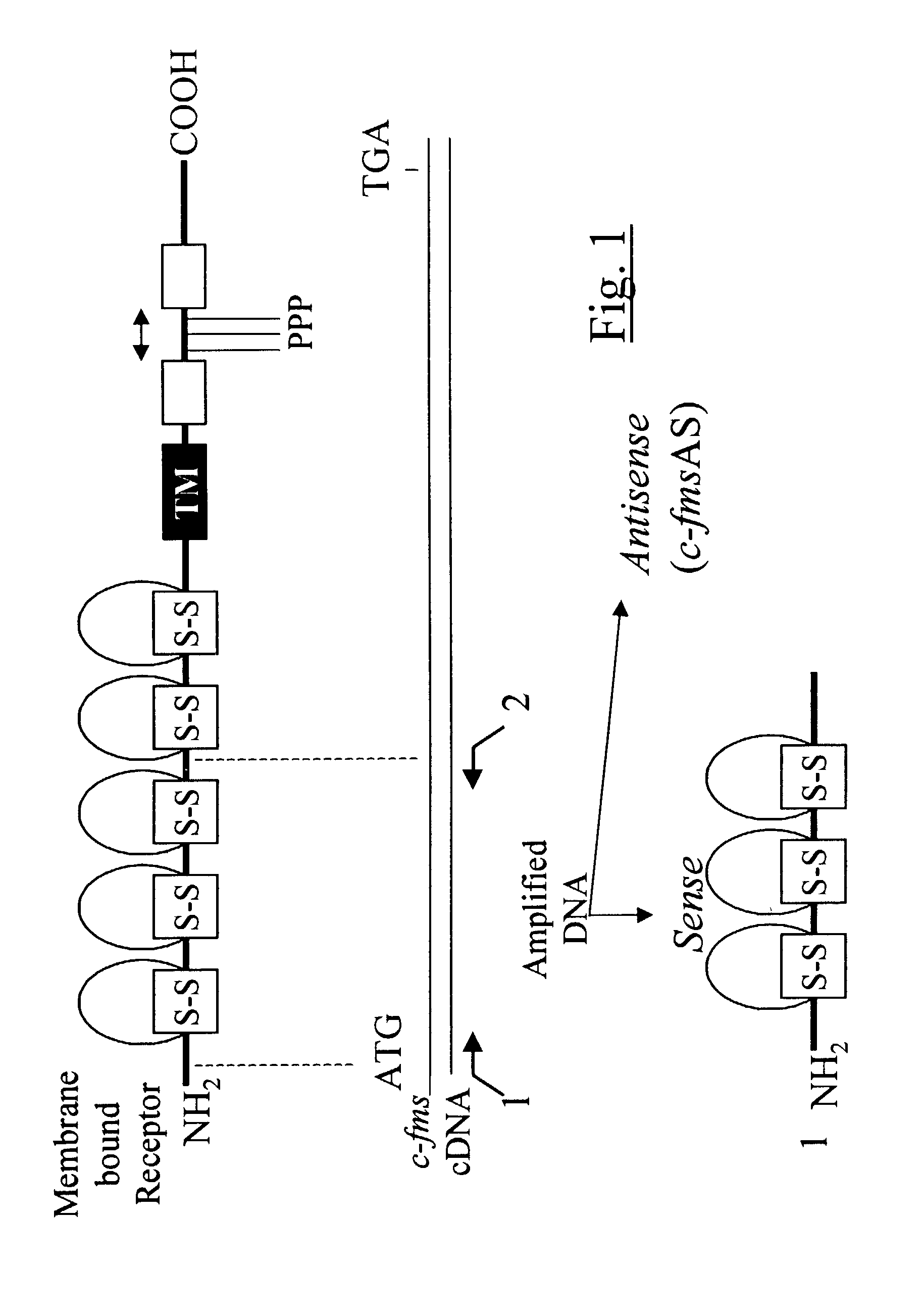
Methods for inhibiting macrophage colony stimulating factor and c-FMS-dependent cell signaling
Owner:RAJAVASHISTH TRIPATHI
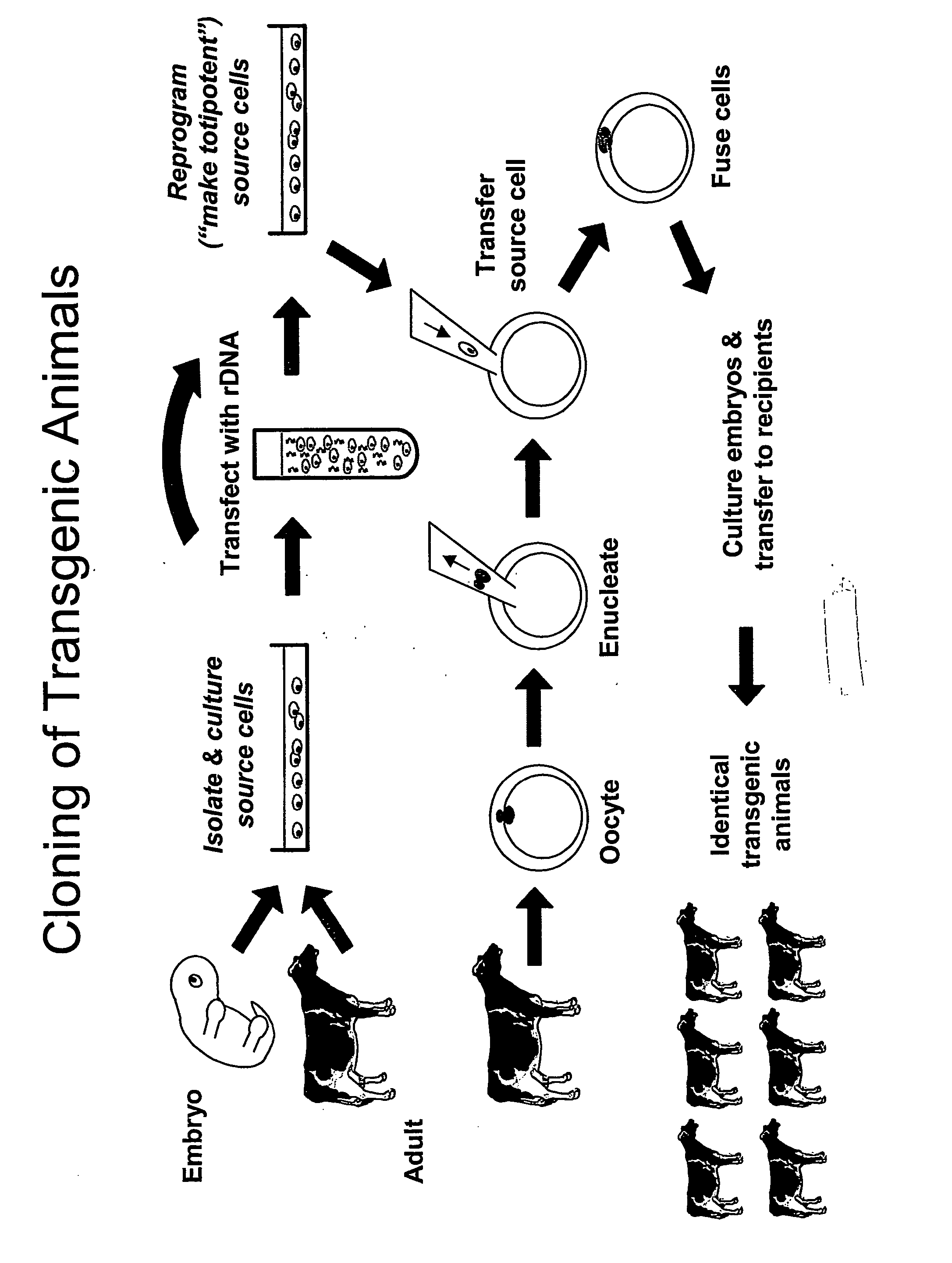
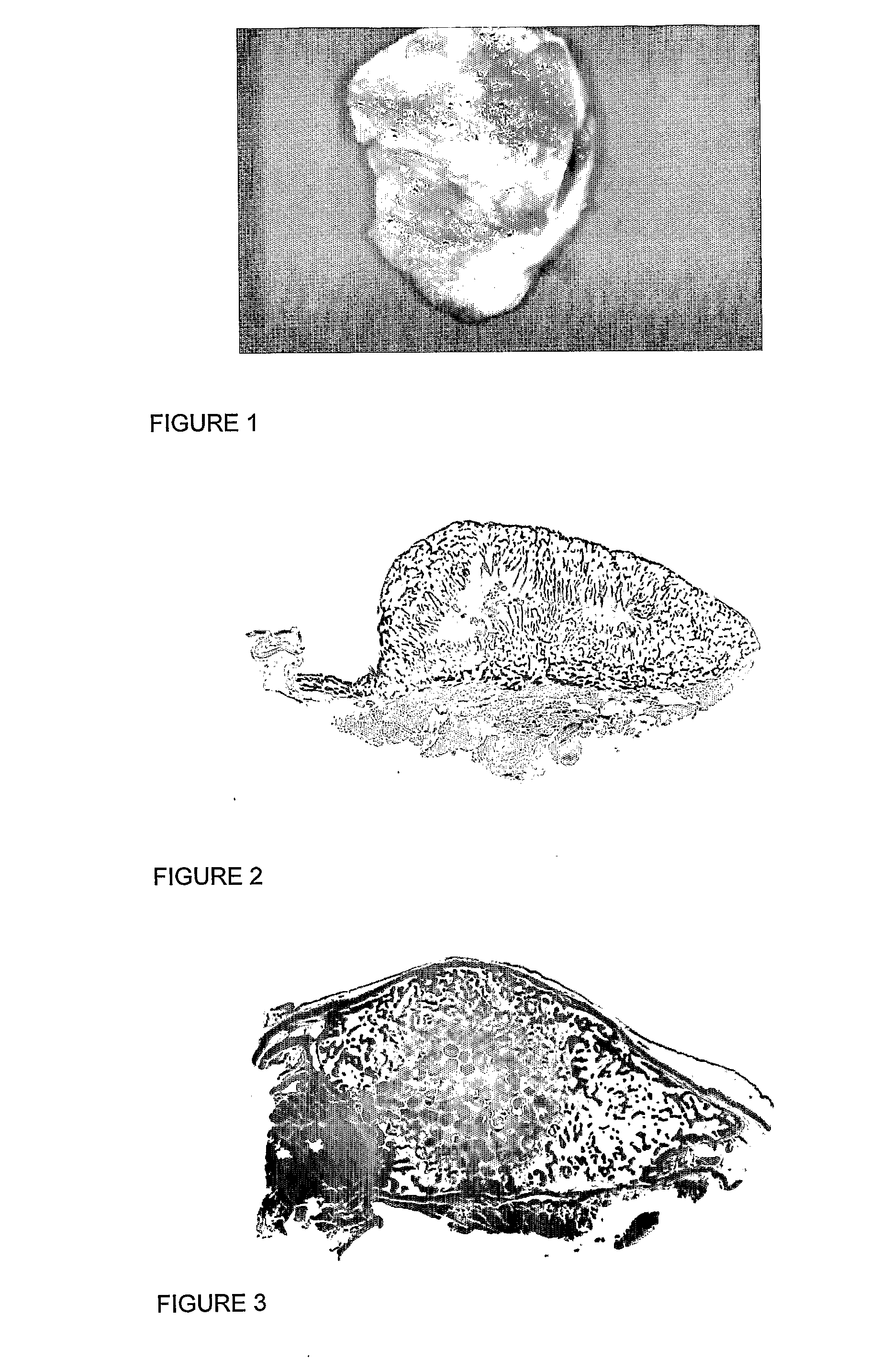
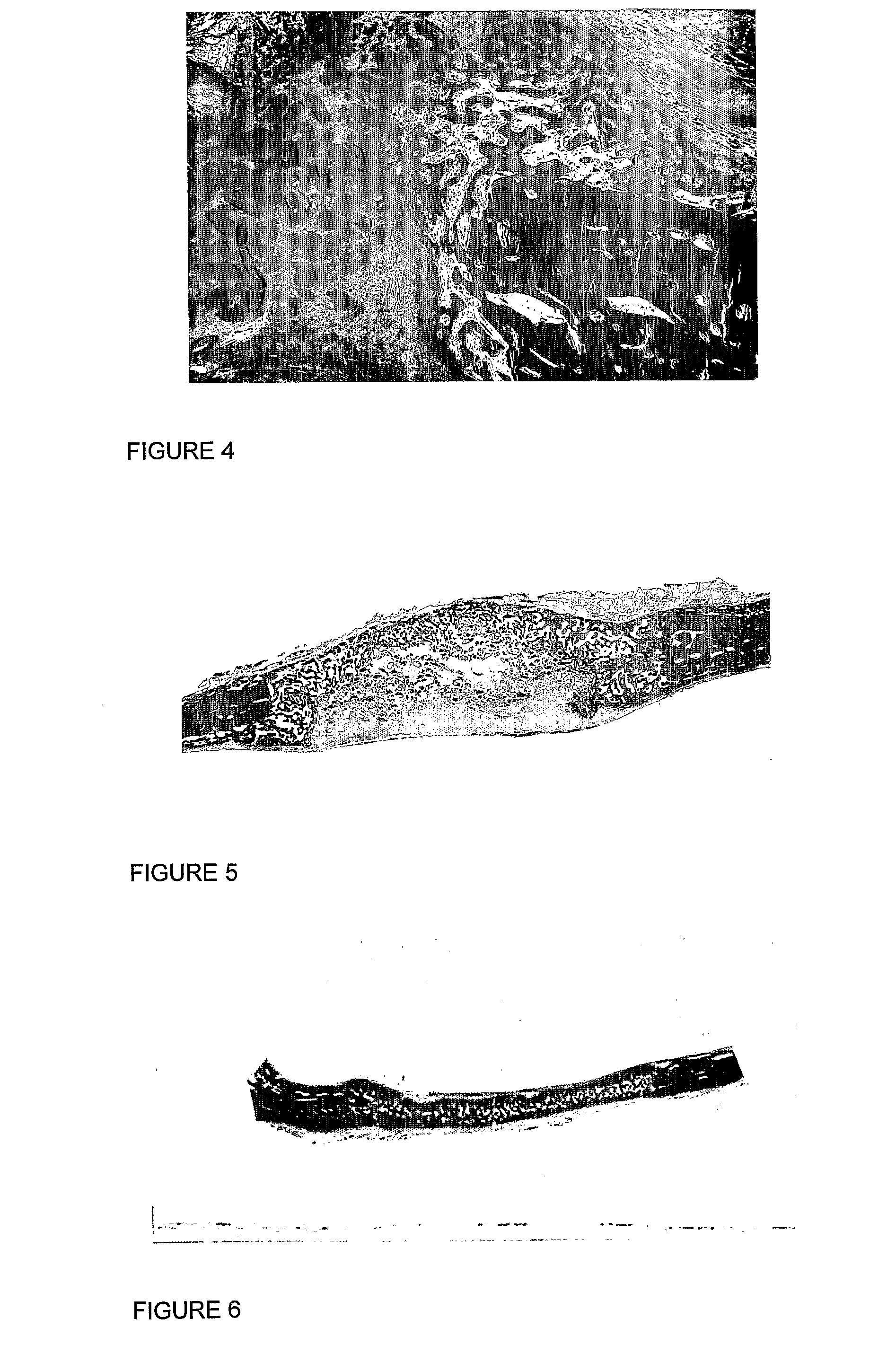
Method and system for fusion and activation following nuclear transfer in reconstructed embryos
InactiveUS20050177882A1Improve usabilityPrevent rejectionNew breed animal cellsRecombinant DNA-technologyActivation methodPrimate
Owner:GTC BIOTHERAPEUTICS INC
Method of biological control
Owner:BIOVITE AUSTRALIA PTY LTD
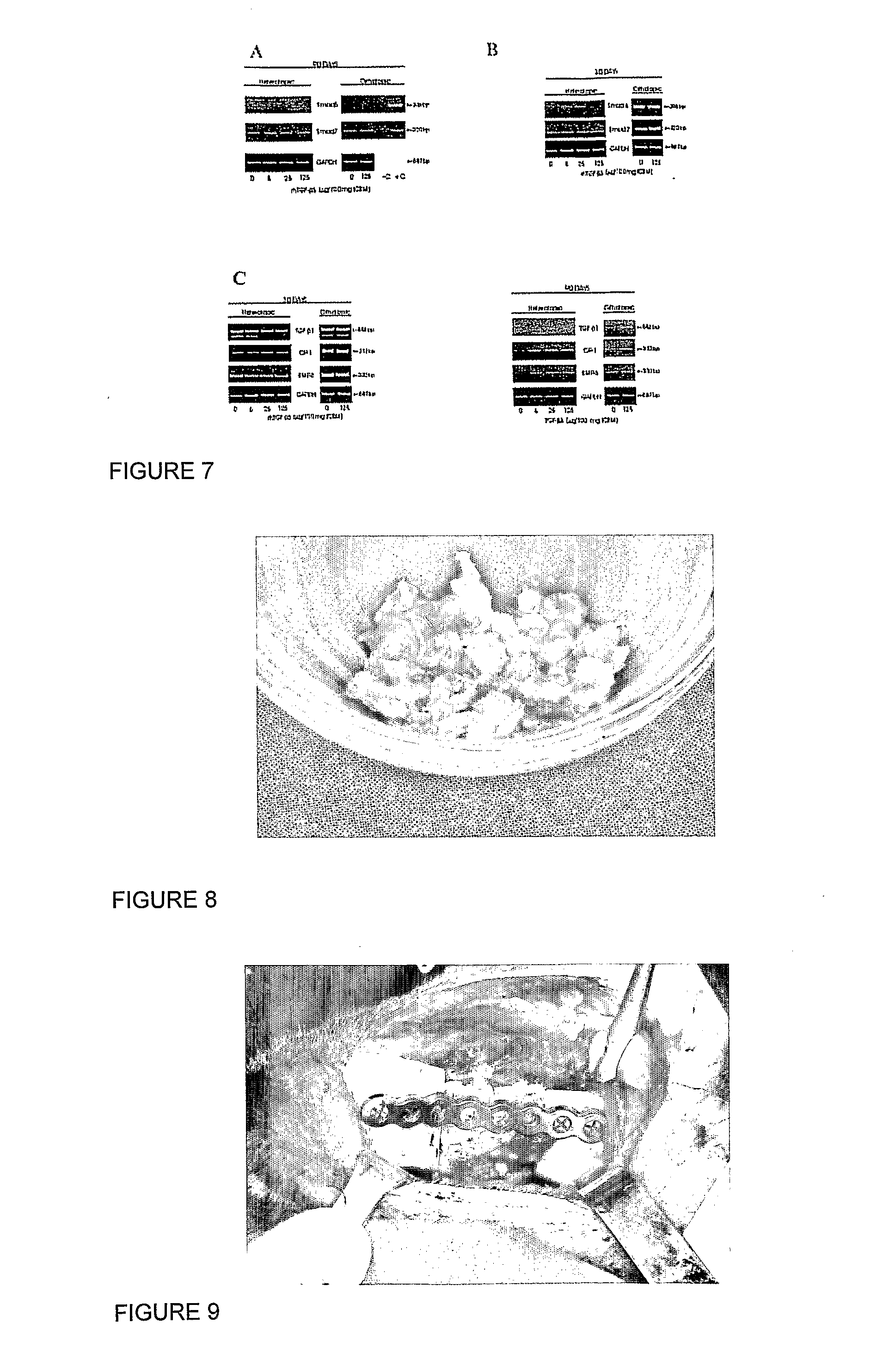
Use of probiotics to ameliorate diet-induced insulin resistance
InactiveUS20120027737A1Ameliorating and preventing diet-induced insulin resistanceHigh expressionBiocideBacteriaBiotechnologyIntestinal structure
The invention relates to the use of a composition comprising probiotic bacteria that regulate expression of key components involved in diet-induced insulin resistance for ameliorating or preventing diet-induced insulin resistance. The use of the probiotic strain and / or a fraction of said strain and / or metabolite of said strain for the manufacture of a medicament or a food or feed product to ameliorate diet-induced insulin resistance and help to obtain optimal body weight of a mammal is disclosed. Preferably, the composition comprises at least one probiotic Lactobacillus acidophilus strain and / or a fraction of said strain and / or metabolite of said strain for ameliorating or preventing diet-induced insulin resistance, said composition characterized by up-regulating expression of the ANGPTL4 gene encoding for FIAF in the intestine, down-regulating expression of the Elovl6 gene in the intestine as well as down-regulating expression of the SCD1 gene in skeletal muscles of a mammal, and wherein the probiotic strain is selected from the group of strains consisting of Lactobacillus acidophilus strain LA-5 (DSM13241).
Owner:CHR HANSEN AS
Osteogenic Device for Inducing Bone Formation in Clinical Contexts
InactiveUS20100068239A1Generation is still usablePowder deliveryPeptide/protein ingredientsMammalBone formation
Owner:UNIVERSITY OF THE WITWATERSRAND +2
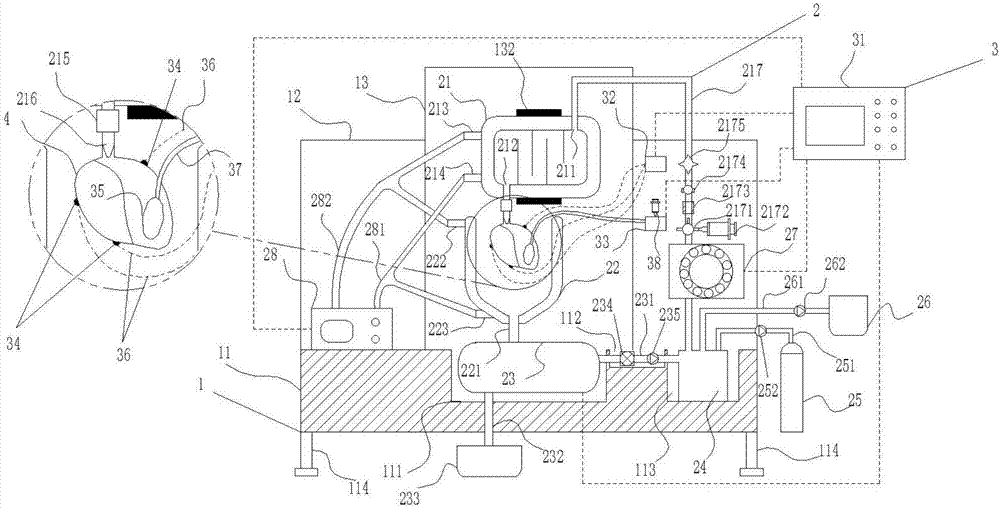
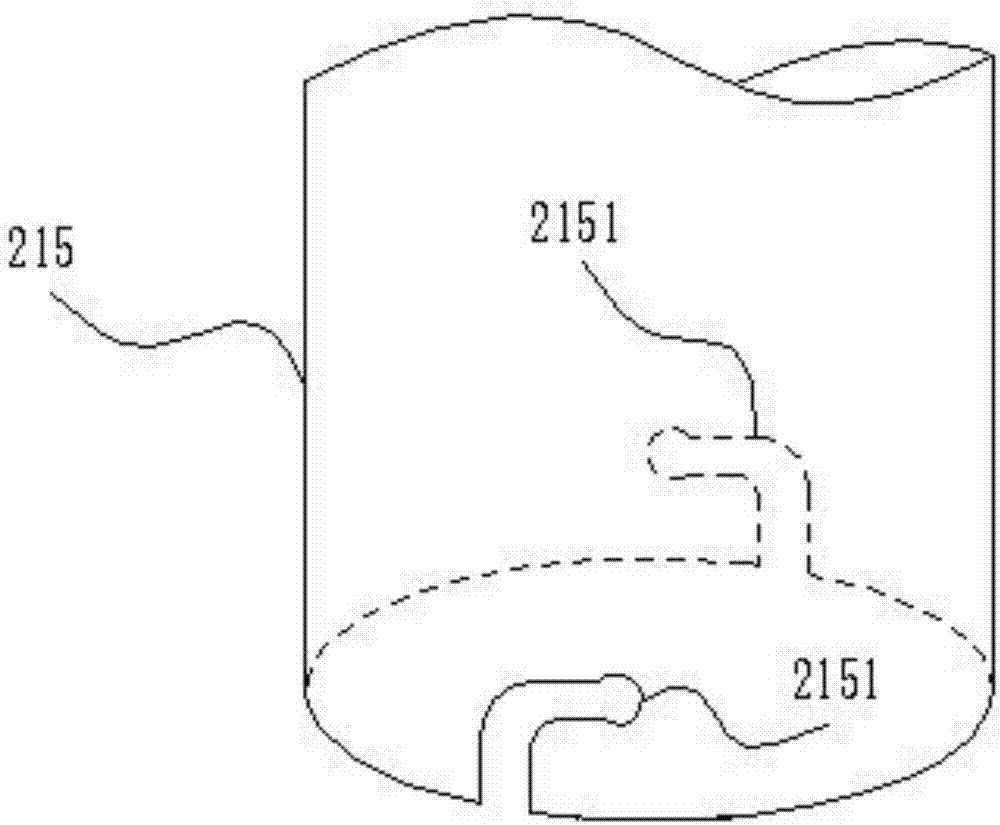
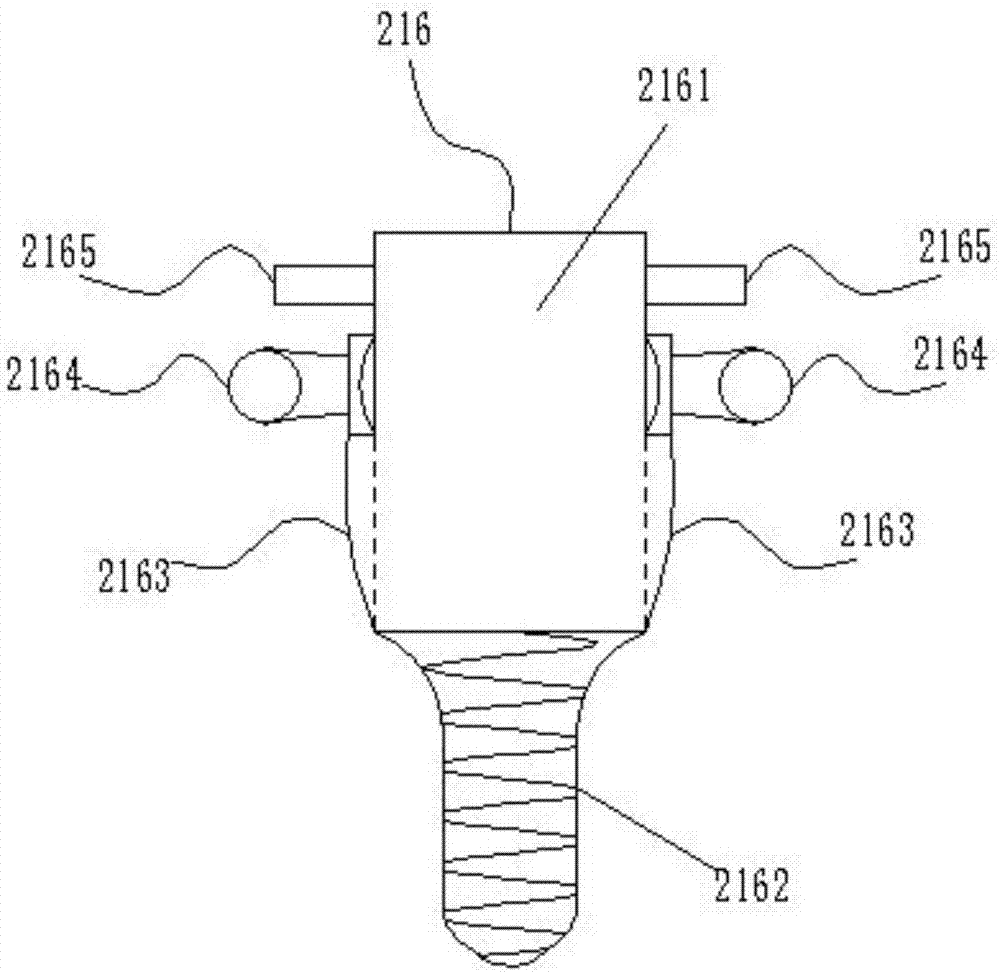
In-vitro heart constant pressure/constant flow perfusion experiment device for cardiological mammals
Owner:镇江市达康医疗器械有限公司
Immortalization of Mammalian Cells
Owner:ROBERTS THOMAS +3
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap