Lipid droplet probe with high selectivity and large Stokes shift as well as preparation method and application of lipid droplet probe
A highly selective, displacement technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of strong fluorescence background, poor selectivity, small Stokes displacement, etc., to achieve simple steps, convenient operation, and dyeing speed. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
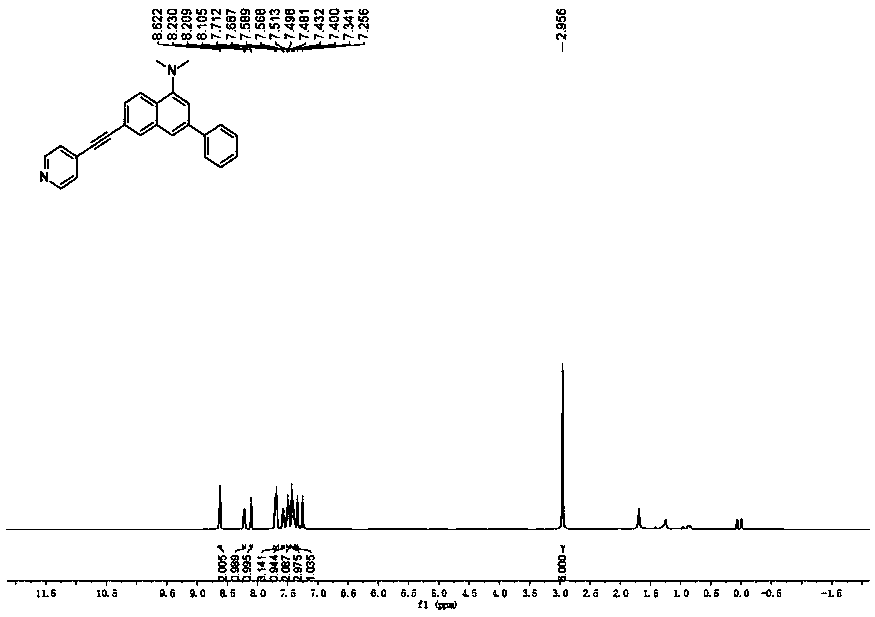
[0031] Synthesis of Probe N,N-Dimethyl-3-phenyl-6-(pyridin-4-ylethynyl)naphthalene-1-amine
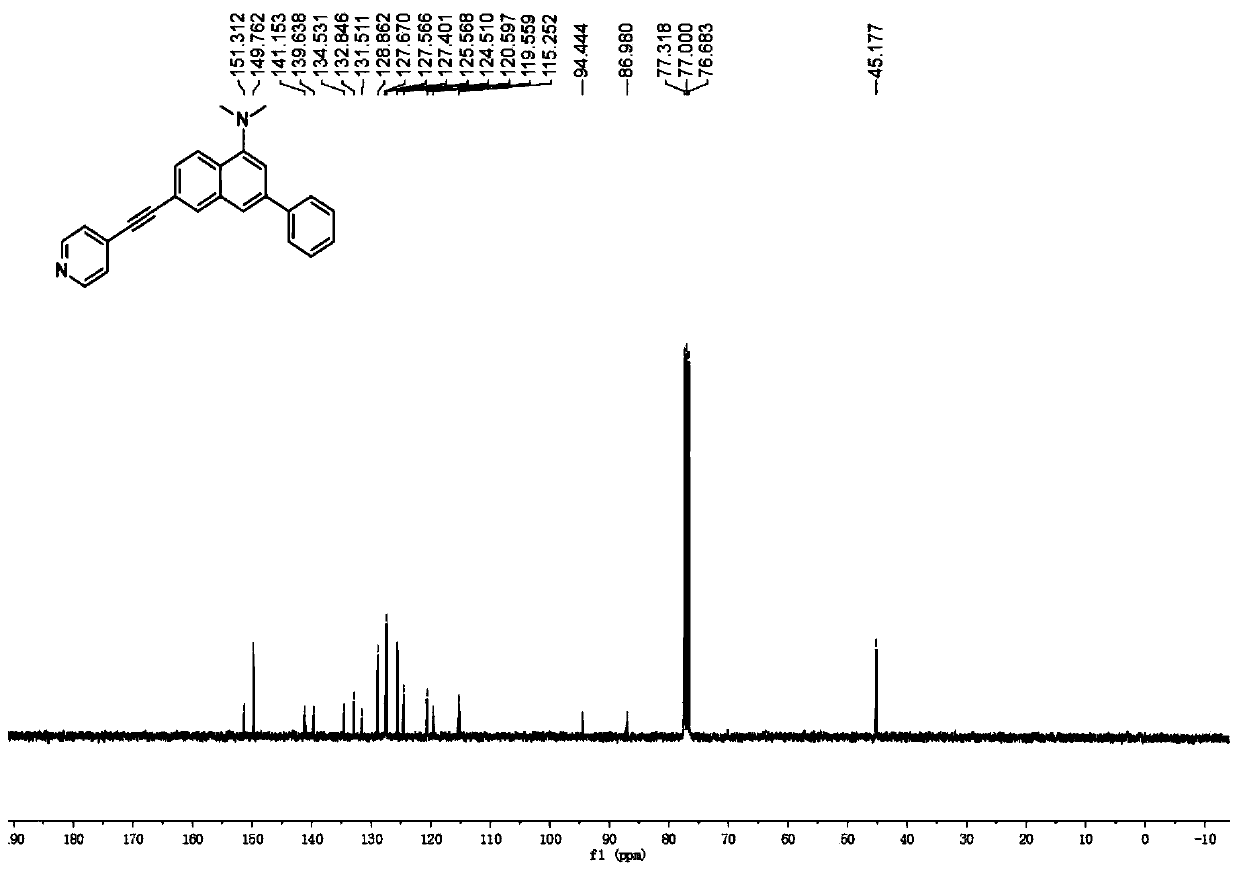
[0032] Add 0.20mmol 6-bromo-N,N-dimethyl-3-phenylnaphthalene-1-amine, 0.40mmol phenylacetylene, 0.01mmol Pd(PPh) to the reactor 3 Cl 2 , 0.01 mmol CuI, 0.40 mmol diisopropylamine, 1.0 mL THF. Under nitrogen atmosphere, heat to 100°C, keep stirring for 30h, stop the reaction, cool to room temperature, add saturated ammonium chloride solution to wash, then extract with dichloromethane, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography The fluorescent probe was obtained with a yield of 88%. 1 H NMR (400MHz, CDCl 3 ):δ8.62(s,2H),8.22(d,J=8.4Hz,1H),8.11(s,1H),7.71–7.68(m,3H),7.58(d,J=8.1Hz,1H) ,7.50(d,J=6.5Hz,2H),7.43–7.40(m,3H),7.34(s,1H),2.96(s,6H); 13 C NMR (100MHz, CDCl 3 ): δ151.3, 149.8, 141.1, 139.6, 134.5, 132.8, 131.5, 128.9, 127.7, 127.6, 127.4, 125.6, 124.5, 120.6, 119.6, 115.2, 94.4, 86.9, 45.2.
Embodiment 2
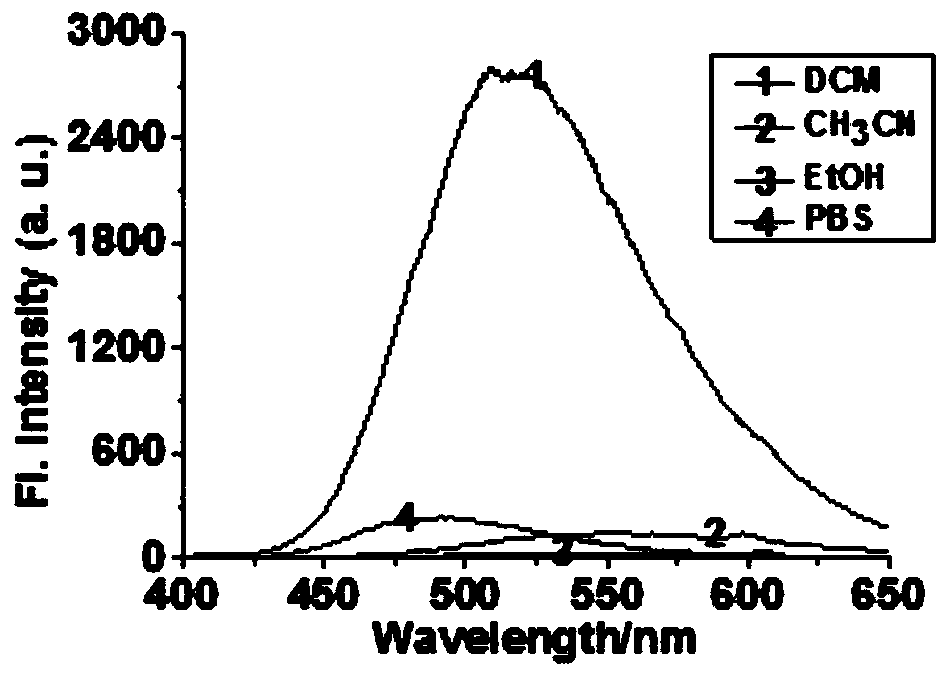
[0034] Emission Spectra of Fluorescent Probes in Different Solvents
[0035] Prepare the fluorescent probe N,N-dimethyl-3-phenyl-6-(pyridin-4-ylethynyl)naphthalene-1-amine synthesized in embodiment 1 at a concentration of 1mM in dichloromethane (DCM) Test the mother liquor for use.
[0036] In the test solution, take 3mL solvents of different polarities: dichloromethane (DCM), acetonitrile, ethanol, PBS buffer solution, and then add 30uL of the probe mother solution, so that the concentration of the probe in the test solution is 10uL, and perform fluorescence Scanning (excitation wavelength 405nm, detection wavelength band 400–650nm) to obtain the fluorescence intensity in each system, such as image 3 As shown, with the increase of polar solvent, the spectrum red-shifted and the fluorescence decreased obviously.
Embodiment 3
[0038] Colocalization of Fluorescent Probes with Commercial Ester Droplet Probes
[0039] Dichloromethane (DCM) of N,N-dimethyl-3-phenyl-6-(pyridin-4-ylethynyl)naphthalene-1-amine of the fluorescent probe synthesized in Example 1 with a concentration of 1mM The test mother solution is ready for use; the test mother solution of dichloromethane (DCM) of commercially available Nile Red (special locator for ester drops) with a concentration of 1 mM is prepared for use.
[0040] HepG2 cells that have been cultured, after the cells adhere to the wall, add the polarity-sensitive fluorescent probe N,N-dimethyl-3-phenyl-6-(pyridin-4-ylethynyl) of the present invention respectively Naphthalene-1-amine 10uL and commercially available Nile Red (special locator for ester drops) solution 5uL, discard the medium, wash the cells with PBS buffer 3 times, then perform fluorescence imaging (excitation wavelength of the probe: 405nm , emission band: 450–550nm; excitation wavelength of Nile Red: ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap