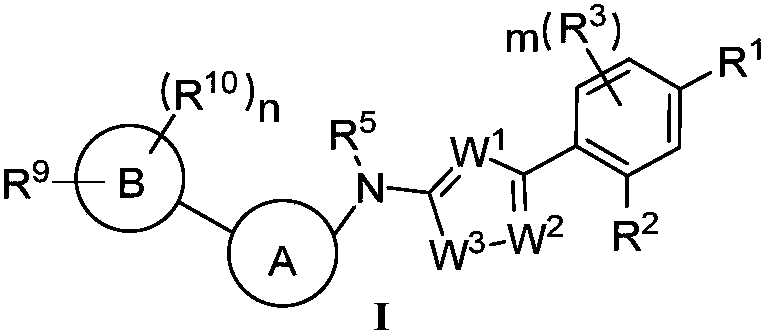
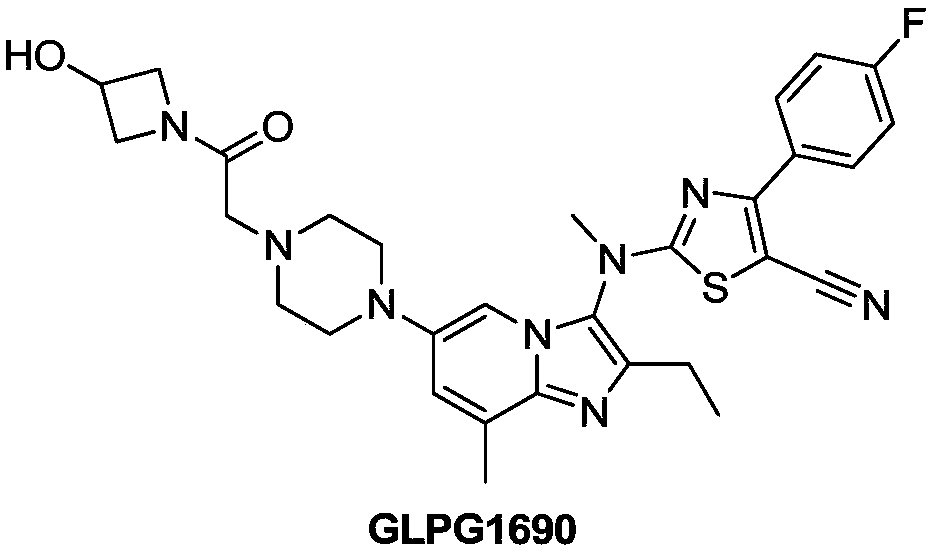
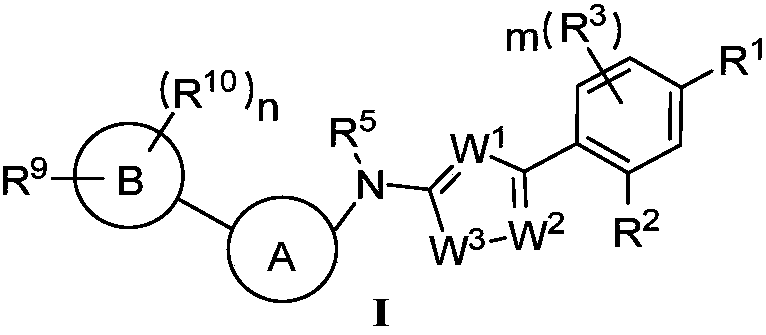
Heterocyclic compound and application thereof and pharmaceutical composition containing heterocyclic compound
A technology of heterocyclic compounds and pharmacy, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, antipyretics, etc., can solve the problem of single structure of ATX inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0824] Preparation Example 1 Intermediate Synthetic Method
[0825]
[0826] Step 1, 2-amino-4-(3,4-difluorophenyl)thiazole-5-carbonitrile
[0827] To a solution of 3-(3,4-difluorophenyl)-3-oxopropionitrile (1.38 g, 7.63 mmol) in ethanol (18 mL) was added pyridine (0.60 g, 7.63 mmol). The reaction was stirred at 70°C for 15 minutes, then cooled to room temperature. Thiourea (1.18g, 215.52mmol) and iodine (1.96g, 7.63mmol) were mixed in ethanol (18mL), and the mixture was slowly added dropwise to the above solution, and stirred at room temperature for 1 hour. The reaction was quenched by adding cold sodium thiosulfate solution (10 mL). After filtering and washing the filter cake with water, the filter cake was dried to give 2-amino-4-(3,4-fluorophenyl)thiazole-5-carbonitrile as an off-white solid (1.50 g, 82.9%). 1H NMR (400MHz, DMSO-d6) δ8.28(s, 2H), 7.89–7.83(m,1H), 7.82–7.78(m,1H), 7.65-7.58(m,1H)
[0828] Step 2, 2-Chloro-4-(3,4-difluorophenyl)thiazole-5-carbonitrile...
Embodiment 1
[0845] Example 1S-0021: 2-((2-ethyl-6-(4-(2-(3-hydroxyazetidin-1-yl)-2-oxoethyl)piperazine-1- Base) imidazo[1,2-a]pyrimidin-3-yl)(methyl)amino)-4-(4-fluorophenyl)thiazole-5-carbonitrile
[0846]
[0847] Step 1: 2-Chloro-1-(3-hydroxyazetidin-1-yl)acetyl
[0848] To an aqueous solution (8 mL) of potassium carbonate (3 g, 22 mmol) was added 3-hydroxyazetidine hydrochloride (1.1 g, 10 mmol). The reaction solution was stirred at room temperature for 10 minutes, then dichloromethane was added to dilute (8 mL) and the reaction solution was cooled to 0°C, and 2-chloroacetyl chloride (1.3 g, 12 mmol) was slowly added dropwise. The reaction solution was stirred at room temperature for 2 hours, filtered, the organic phase was separated, and the aqueous phase was extracted with methanol / ethyl acetate mixture (1 / 1) (20 mL x 6). The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was resuspended in acetone (50 mL) and stirred ...
Embodiment 2
[0867] Example 2S-0022: 2-((2-ethyl-5-(4-(2-(3-hydroxyazetidin-1-yl)-2-oxoethyl)piperazine-1- Base)-7-methyl-2H-indazol-3-yl)(methyl)amine)-4-(4-fluorophenyl)thiazole-5-carbonitrile
[0868]
[0869] Step 1: 5-Bromo-2-fluoro-3-methylbenzonitrile
[0870] Add iodine (11.67g, 46.3mmol) to a mixture of 5-bromo-2-fluoro-3-methylbenzaldehyde (5g, 23.1mmol) in tetrahydrofuran (20mL) and ammonia water (20mL), and the reaction solution was heated at room temperature Stir for 16 hours. Sodium bisulfite solution (20 mL) was added to the reaction solution to quench the reaction, and then extracted with ethyl acetate (50×2 mL). The organic phases were combined, washed with saturated brine (10 mL x 2), dried over anhydrous sodium sulfate, filtered and concentrated to give 5-bromo-2-fluoro-3-methylbenzonitrile as a white solid (5 g, 98%) , used directly in the next reaction without further purification. 1 H NMR (400MHz, CDCl 3 )δ7.55-7.59(m,2H),2.32(s,3H).
[0871] Step 2: 5-Bromo-...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap