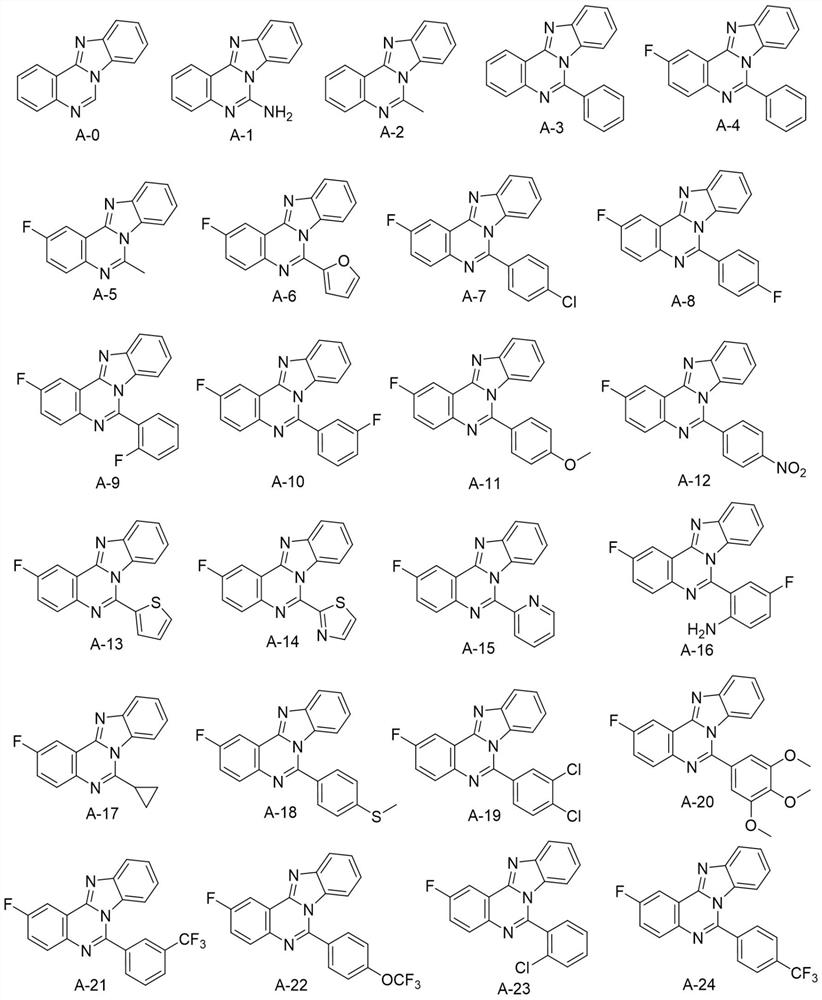
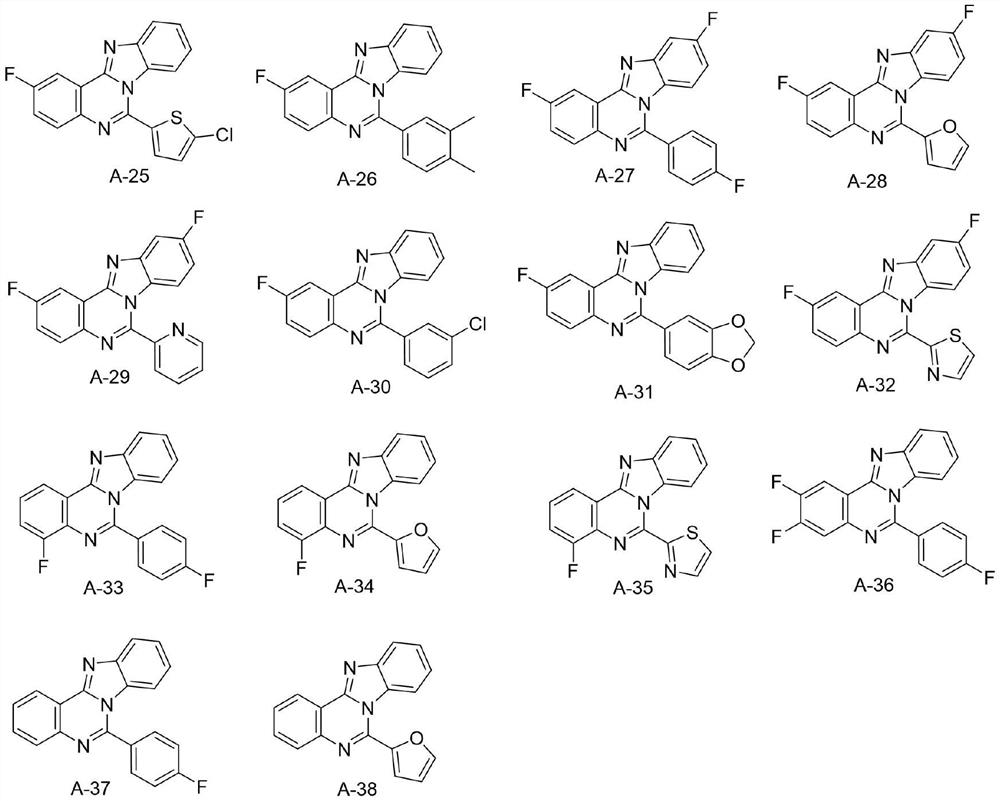
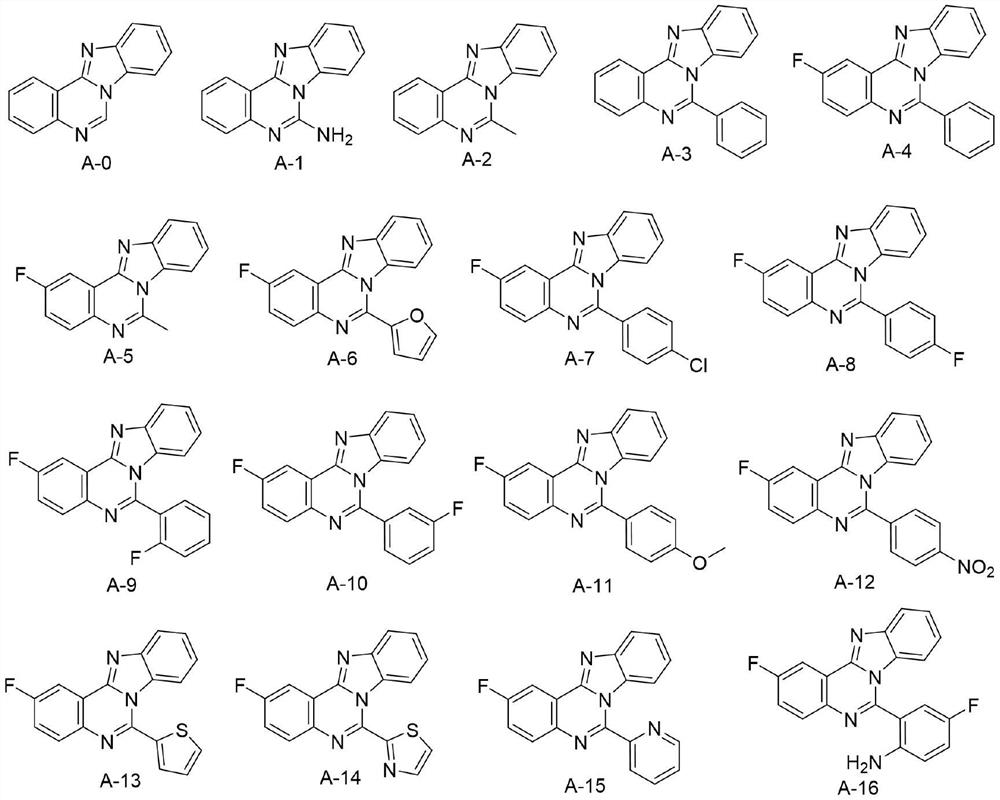
Application of Aza-type iso-cryptolepine derivatives in prevention and treatment of agricultural plant diseases
A technology of isoftenine and semenine, which is applied in the field of medicinal chemistry and can solve the problems of an alarming growth rate of resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the synthesis of target compound A-0
[0019]
[0020] The synthetic method of compound A-0 of the present invention is carried out according to the following reaction formula:
[0021]
[0022] Synthesis of target compound A-0: 2-(2 bromophenyl)benzimidazole (1mmol), sodium azide (2mmol), cuprous iodide (0.2mmol), p-toluenesulfonic acid (2mmol), tert Add butyl hydroperoxide (1mmol) into a 50mL round-bottomed flask, then add an appropriate amount of N,N-dimethylacetamide (DMA), reflux at 130°C for 10h under nitrogen protection, cool to room temperature after reaction, add dichloro 50mL of methane was extracted several times with brine and water, the organic layer was taken, spin-dried, and purified by column chromatography to obtain it (refer to the literature for the synthesis method: Eur.J.Org.Chem., 2017, 514-522).
[0023] Yield: 67.5%; light yellow powdery solid; m.p.: 228.3-230.6°C; 1 H NMR (400MHz, Chloroform-d 6 )δ9.15(s,1H),8.68(dd,J=7.9,...
Embodiment 2
[0024] Embodiment 2: the synthesis of target compound A-1
[0025]
[0026] The synthetic method of compound A-1 of the present invention is carried out according to the following reaction formula:
[0027]
[0028] The synthesis of target compound A-1: get 2-(2-bromophenyl) benzimidazole derivative (2mmol), guanidine hydrochloride (3mmol), cuprous iodide (5mol%) and potassium phosphate (6mmol) respectively Add 7 mL of DMF to a 50 mL round-bottomed flask, blow nitrogen gas three times, exhaust the air, reflux at 100°C for 16 h, and monitor the reaction by TLC. After the reaction was completed, cool to room temperature, dissolve with 50mL of dichloromethane, add 150mL of saturated sodium chloride solution for extraction three times, then wash the organic phase with water, collect the organic layer, evaporate to dryness under reduced pressure, and dissolve with petroleum ether-ethyl acetate The system was purified by silica gel column chromatography to obtain . (Refer ...
Embodiment 3
[0030] Embodiment 3: the synthesis of target compound A-2
[0031]
[0032] The synthesis method is the same as in Example 2, only replacing guanidine hydrochloride with amidine hydrochloride.
[0033] Yield: 48.0%; pale yellow powdery solid; m.p.: 200.0-202.3°C; 1 H NMR (400MHz, DMSO-d 6 )δ8.40(dd, J=7.9,1.6Hz,1H),7.92(d,J=8.0Hz,1H),7.66(ddd,J=8.5,7.0,1.7Hz,1H),7.58–7.51(m ,2H),7.46(s,2H),7.40-7.35(m,1H),3.18(s,3H). 13C NMR (101MHz, DMSO-d6) δ148.90, 146.96, 145.11, 144.31, 132.27, 128.63, 125.52, 124.82, 124.24, 123.63, 122.80, 119.52, 115.12, 114.79, 19.12 / z: CESI m 15 h 11 N 3 : 234.14[M+H] + .
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap