Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
10 results about "Immunogenicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunogenicity is the ability of a particular substance, such as an antigen or epitope, to provoke an immune response in the body of a human and other animal. In other words, immunogenicity is the ability to induce a humoral and/or cell-mediated immune responses.
Monocytic leukemia associated antigen MLAA-34 resistant fully human monoclonal single-chain antibody ScFv
ActiveCN105801700AAvoiding Immunogenicity IssuesSmall molecular weightImmunoglobulins against cell receptors/antigens/surface-determinantsFermentationSingle-Chain AntibodiesHeavy chain
Owner:THE SECOND AFFILIATED HOSPITAL OF XIAN JIAOTONG UNIV
Immunogenic Treatment Of Cancer
InactiveUS20140356397A1Bacterial antigen ingredientsElectrotherapyRadiofrequency ablationRadiation exposure
Owner:IMMODULON THERAPEUTICS
Oral cavity defect repair membrane and preparation method thereof
PendingCN109260518AHigh biosecurityLow immunogenicityTissue regenerationProsthesisDefect repairAcellular matrix
Owner:广州聚明生物科技有限公司
Recombinant adenovirus rAd-ORF2-TCE and application thereof
InactiveCN103805573AEnhance humoral immunityEnhance cellular immunityGenetic material ingredientsMicroorganism based processesPorcine circovirusOrganism
The invention relates to a recombinant adenovirus rAd-ORF2-TCE. The recombinant adenovirus rAd-ORF2-TCE is obtained through fusing and connecting an immunogenic protein gene ORF2 of PCV2 (Porcine Circovirus Type 2) and three T lymphocyte epitope (TCE) series genes, and then, recombining to an adenovirus vector. The invention further relates to application of the recombinant adenovirus rAd-ORF2-TCE in porcine viral immunization vaccines. According to the recombinant adenovirus rAd-ORF2-TCE disclosed by the invention, an organism can be effectively stimulated to generate a high-level specific antibody and generate T lymphocyte proliferation, and both the concentration of IFN-gamma in blood serum and the concentration of IL-2 in the blood serum are increased remarkably; compared with ORF2 of separate PCV2, the recombinant adenovirus rAd-ORF2-TCE has the advantage that both humoral immunity level and cellular immunity level of an animal body are increased remarkably.
Owner:GUANGDONG WENS DAHUANONG BIOTECH +1
Soluble hiv-1 envelope glycoprotein trimers
ActiveUS20170035877A1Viral antigen ingredientsVirus peptidesHiv 1 vaccineHiv 1 envelope
The present application relates to novel HIV-1 envelope glycoproteins which may be utilized as an HIV-1 vaccine immunogens, antigens for crystallization and for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE +1
Preparation method and application of taenia pisiformis 18kDa antigen
InactiveCN103074344AImproving immunogenicityFermentationAnimals/human peptidesRapid amplification of cDNA endsTotal rna
The invention belongs to the technical field of biology of genes, and provides a preparation method and an application of a taenia pisiformis 18k Da antigen. Taenia pisiformis hexacanth embryos are activated and subjected to total RNA (Ribose Nucleic Acid) extraction; the total RNA is taken as a template to synthetize a cDNA; a Tp18 code area is amplified, and the Tp18 gene bioinformatics analysis is carried out; the prokaryotic expression is performed on the Tp18, and the expression protein is purified. According to the preparation method, the Tp18 genes are amplified from the activated aenia pisiformis hexacanth embryos by an RACE (rapid-amplification of cDNA ends) PCR (Polymerase Chain Reaction) technology to construct a pET32a / Tp18 expression vector, the recombination protein is obtained through IPTG induction, and the obtained recombination protein has better immunogenicity, can serve as a candidate antigen of a taenia pisiformis vaccine, and has stronger popularization and application values.
Owner:SICHUAN AGRI UNIV
Nucleic acid aptamer capable of detecting human colon cancer and application thereof in preparing detection preparations
ActiveCN109628455AAccurate diagnosisRapid positioningMaterial analysisDNA/RNA fragmentationChemical synthesisAptamer
The invention discloses a nucleic acid aptamer capable of achieving targeted detection of colon cancer cells and application thereof. The nucleotide sequence of the nucleic acid aptamer is 5'-ACGCTCGGATGCCACTACACGGTTGGGGTCGGGCATGCGTCCGGAGAAGGGCAAACGAGAGGTCACCAGCACGTCCATGAG-3'. The nucleic acid aptamer is good in stability, target molecules can be specifically identified, the immunogenicity in thebody is small, and the target molecules can be easily removed. The aptamer is small in molecular weight and low in preparation cost, can be obtained through chemical synthesis in vitro, and is easy tostore and transport. By adopting the nucleic acid aptamer, various colon cancer cells can be detected, the operation is simple and quick, and early diagnosis, targeted treatment, prognosis and the like of colon cancer are facilitated.
Owner:HUNAN UNIV
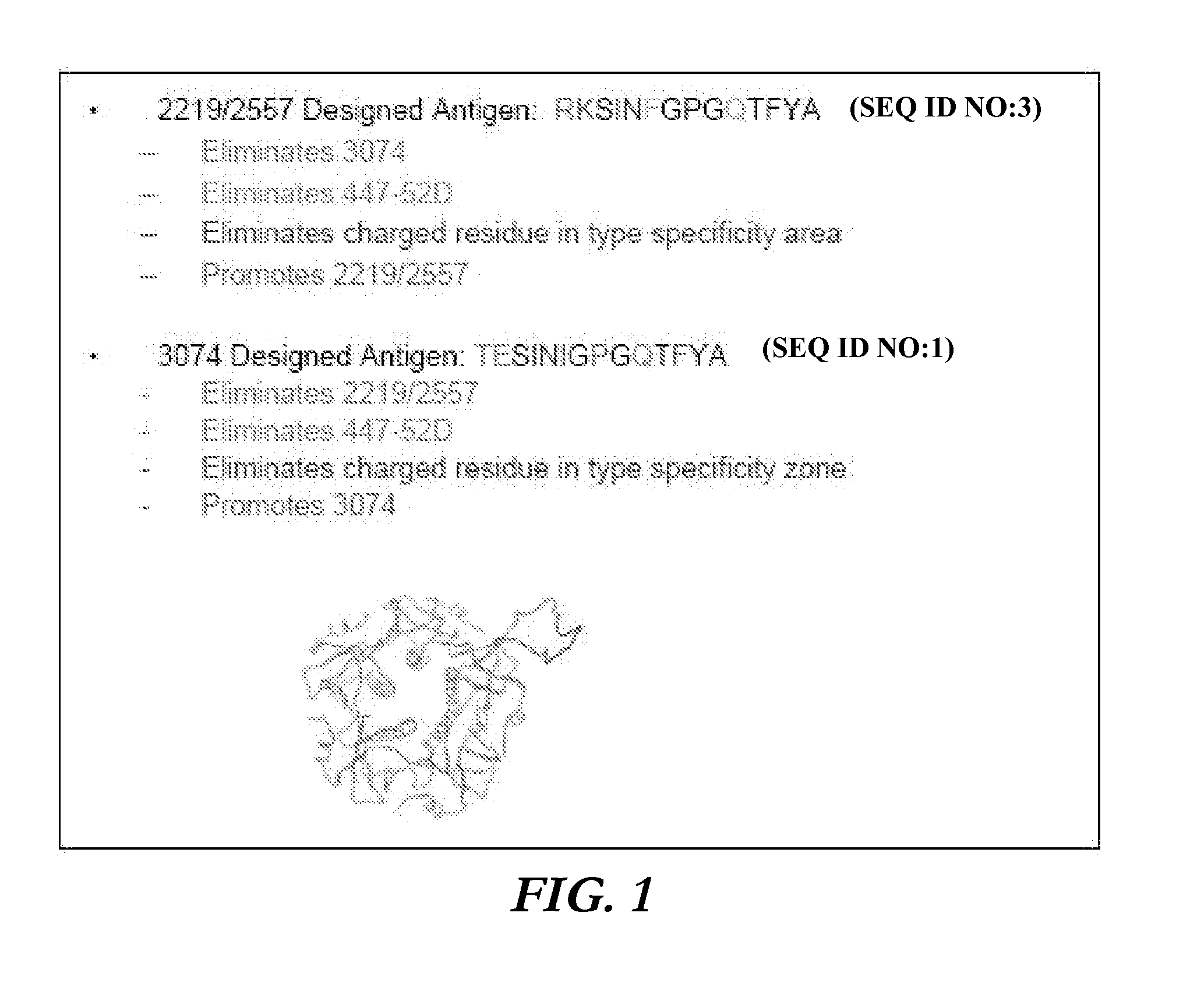
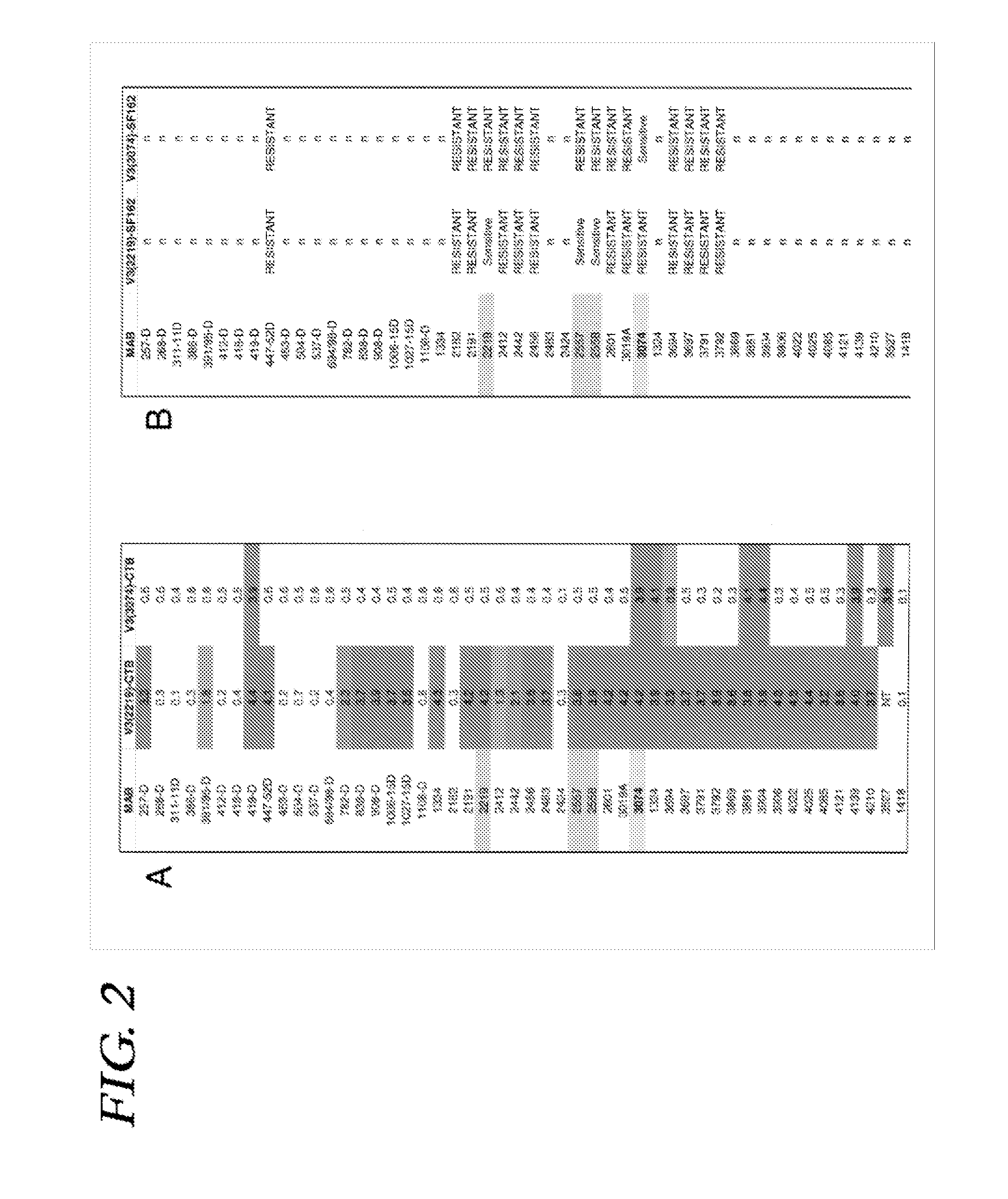
Immunogenic polypeptides having an immunogenic scaffold protein and a loop peptide, presenting a 3074- or 2219/2557- monoclonal antibody-targeted epitope, which is present in the HIV gp120 protein
ActiveUS20130287804A1Bridging the gapPeptide/protein ingredientsVirus peptidesCyclic peptideHeterologous
Owner:MOLSOFT +2
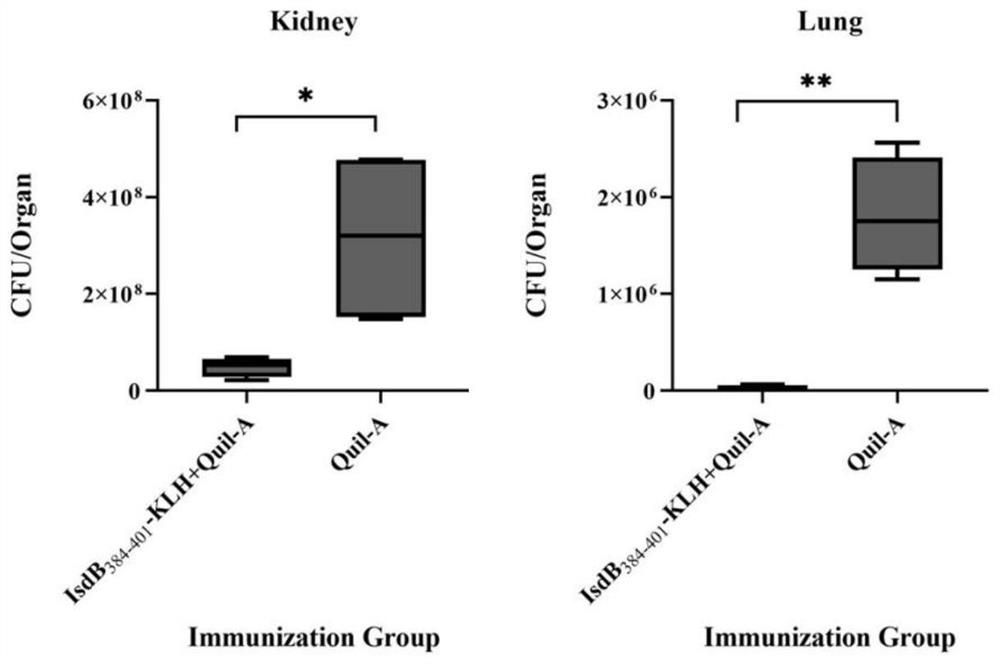
IsdB antigen epitope peptide for diagnosing or preventing staphylococcus aureus infection and application thereof
PendingCN112920259AImprove securityEfficient infectionConnective tissue peptidesAntibacterial agentsAntigen epitopeStaphyloccocus aureus
Owner:ARMY MEDICAL UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap