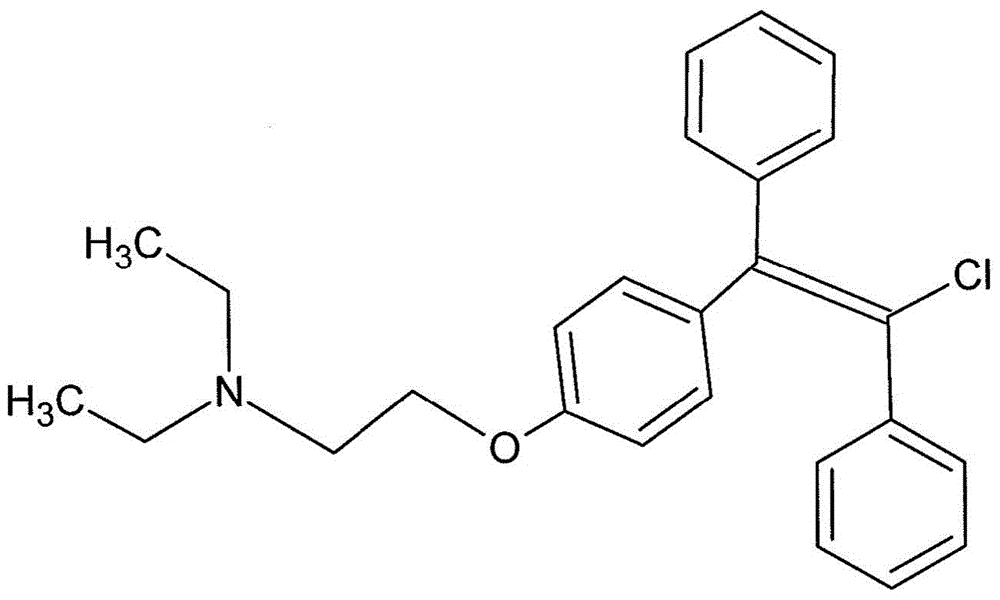
Trans-clomiphene formulations and uses thereof
A technology of trans-clomiphene and clomiphene citrate, which is applied in the field of trans-clomiphene and can solve the problems of genotoxic tumor enhancement effect and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Preparation of trans-clomiphene citrate
[0036] Clomiphene citrate was prepared as follows:
[0037] A mixture of 20 g of 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-diphenylethanol in 200 cc of ethanol containing excess hydrogen chloride was refluxed for 3 hours. The solvent and excess hydrogen chloride were removed in vacuo, and the residue was dissolved in a mixture of ethyl acetate and dichloromethane. 1-[p-(β-Diethylaminoethoxy)phenyl]-1,2-stilbene hydrochloride melting at 148°C to 157°C was obtained. The hydrochloride was treated with N-chlorosuccinimide in anhydrous chloroform at reflux. The product obtained subsequently was converted to free base and treated with citric acid. The dihydrocitrate salt of 1-[p-(β-diethylaminoethoxy)phenyl]-1,2-diphenylchloroethene was obtained melting at 116.5°C to 118°C. The clomiphene citrate obtained by this process contains between 30% and 50% of the cis isomer and between 70% and 50% of the trans isomer.
[0038] Trans-clomip...
example 2
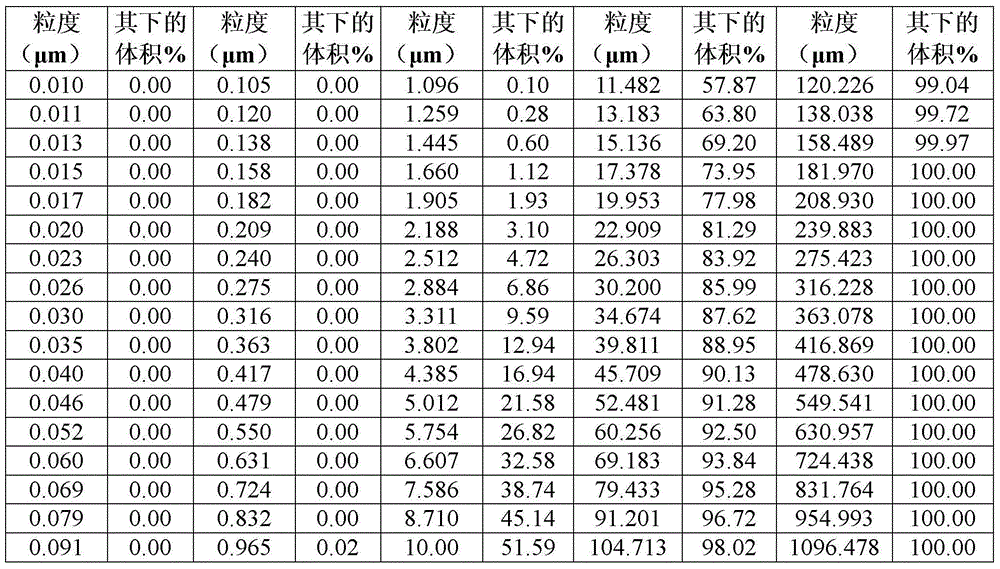
[0040] Particle size analysis
[0041] Particle size characterization of trans-clomiphene is performed using an instrument adapted to measure equivalent spherical volume diameter, such as a Malvern Mastersizer 2000 laser diffraction particle size analyzer or equivalent. After particle size characterization, trans-clomiphene is then milled if necessary, preferably using a pin mill under suitable conditions of mill rotation rate and feed rate to bring the particle size values within the above-mentioned limits according to the invention. Grinding efficiency was checked by sampling using a Malvern Sizer 2000 laser diffraction particle size analyzer and final particle size was checked in a similar manner.
[0042] Trans-clomiphene in microparticle form within the above-mentioned limits according to the invention can then be mixed with excipients or carriers if desired and used eg for filling capsules. Due to the irregular shape of the particles before or after milling, it is nec...
example 3
[0071] formulation
[0072] Gelatin capsules containing trans-clomiphene were prepared using:
[0073] components Quantity (mg / capsule) trans-clomiphene citrate 5.0-100 microcrystalline cellulose 0-343.2 Magnesium stearate 0-8-
[0074] Trans-clomiphene in crystalline form was blended with 1 / 3 of the total microcrystalline cellulose and passed through a mesh screen to ensure good distribution of the material. The remaining 2 / 3 of the microcrystalline cellulose was then passed through a mesh screen and blended with the powder mixture. followed by a suitable milling machine (e.g. mill) to grind the resulting mixture. Magnesium stearate previously passed through a mesh sieve is added and mixed with the resulting granulation. After homogeneity analysis, the resulting mixture was encapsulated into gelatin capsules. A preferred gelatin capsule (size 3) formulation is as follows:
[0075] components Quantity (mg / capsule) trans...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap