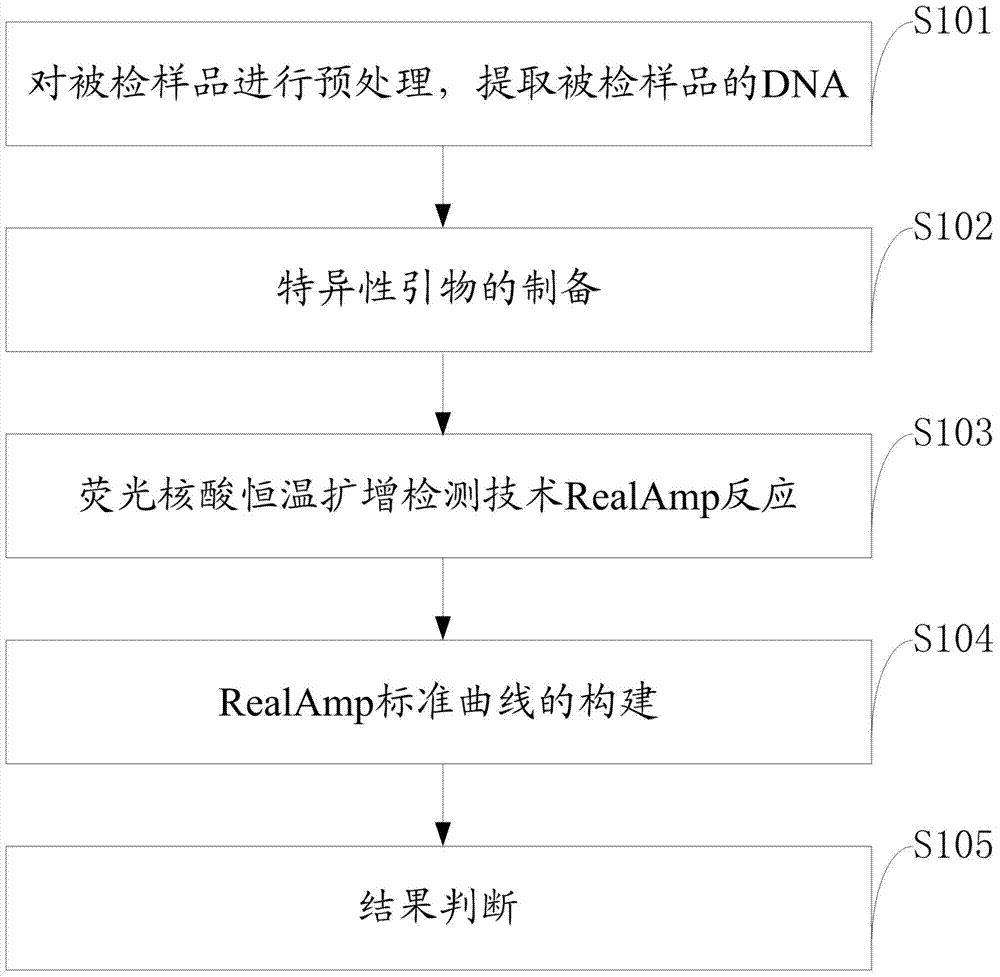
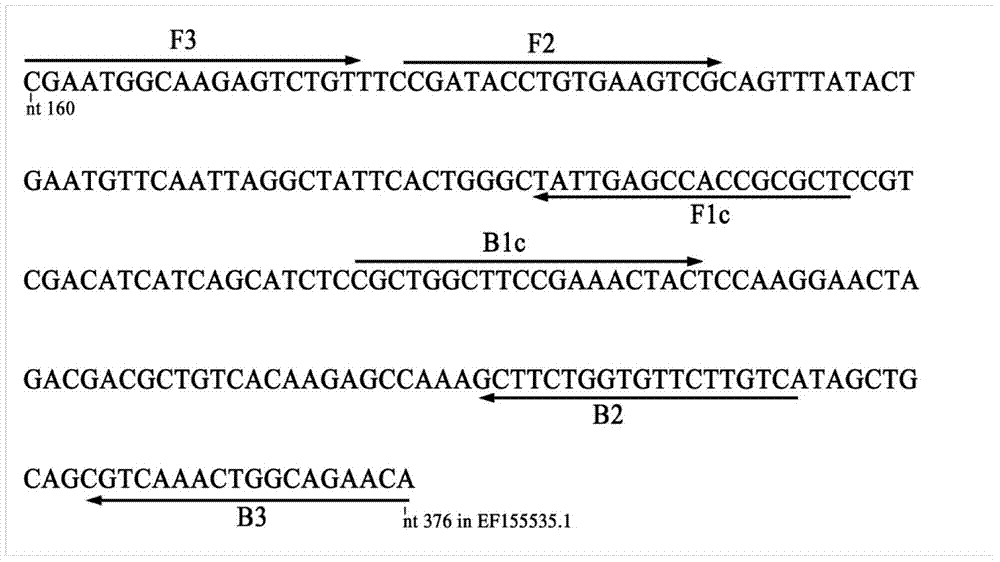
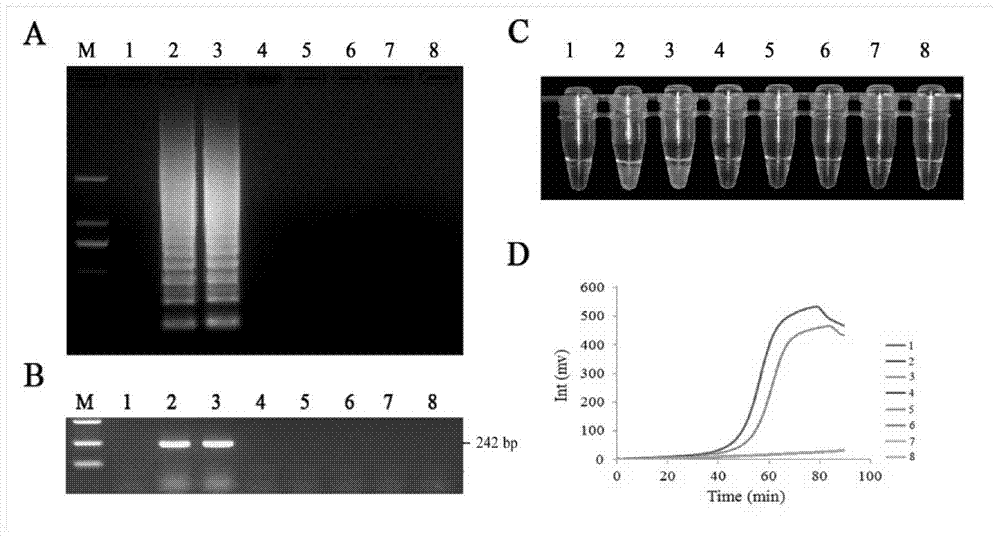
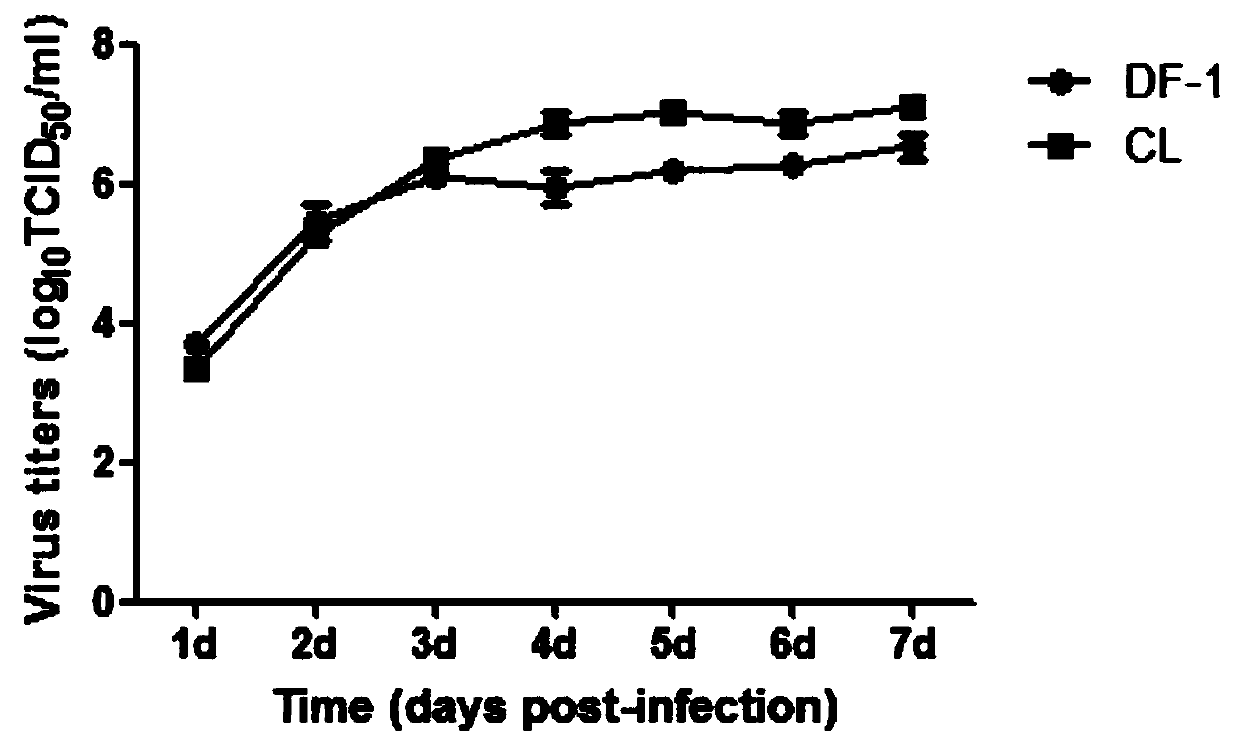
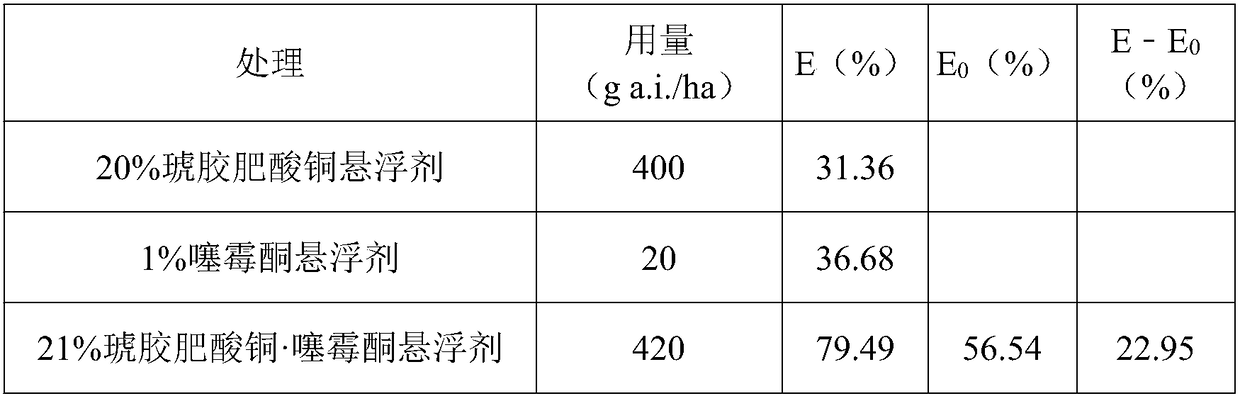
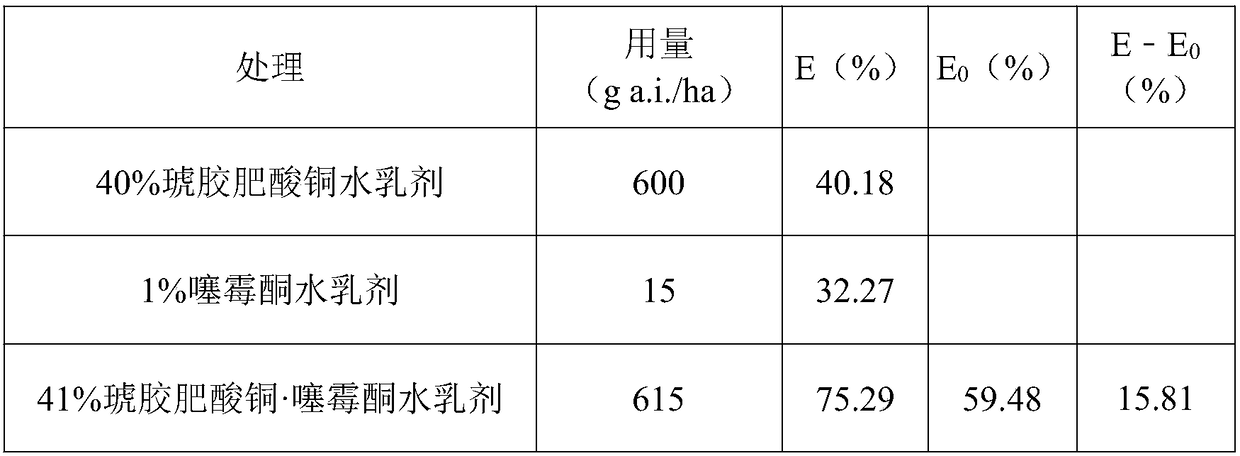
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
18 results about "Pathogen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In biology, a pathogen (Greek: πάθος pathos "suffering", "passion" and -γενής -genēs "producer of") in the oldest and broadest sense, is anything that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ.
Serum-free culture system for efficiently culturing human umbilical cord mesenchymal stem cells in vitro
InactiveCN104164405AGood repeatabilityImprove passaging stabilitySkeletal/connective tissue cellsAntioxidantCytokine
The invention relates to a serum-free culture system for efficiently culturing human umbilical cord mesenchymal stem cells in vitro. The serum-free culture system comprises the following components: a basal culture medium a-MEM, cell factors, hormones and proteins, unsaturated fatty acids, antioxidants, energy substances, an amino acid additive, vitamins and metal additives. The serum-free culture system comprises simple and clear components, is free from harms of pathogens, does not have difference between batches, has good repeatability, can obtain plenty of high-quality human umbilical cord mesenchymal stem cells in a short time and has high passage stability, so that the serum-free culture system can be applied to scientific researches and can provide high-purity vibrant cells for cell therapy as a mating system in cell therapy.
Owner:CYAGEN BIOSCI INC
Screening assays for antimicrobial agents
InactiveUS20050282242A1Reduce biological activityReduced expression levelMicrobiological testing/measurementMicroorganismAntimicrobial drug
Owner:ACTIVBIOTICS INC
Multiplex immuno screening assay
ActiveUS20140274762A1Peptide librariesLibrary screeningPathogenFluorescent microspheres
Owner:INST PASTEUR
Quantitative detection method for FOC race 4 from soil
InactiveCN103757093AHigh sensitivityStrong specificityMicrobiological testing/measurementFusarium oxysporumNucleic acid detection
Owner:ENVIRONMENT & PLANT PROTECTION INST CHINESE ACADEMY OF TROPICAL AGRI SCI
Acne-removing liquid containing tea tree flower extract
InactiveCN106726998ANo side effectsLong-term useSalicyclic acid active ingredientsCosmetic preparationsSide effectAdditive ingredient
Owner:NANJING INST FOR THE COMPREHENSIVE UTILIZATION OF WILD PLANTS CHINA COOP
Method for efficiently amplifying subgroup J avian leukosis virus (ALV)
ActiveCN107603957AReduce separation and detection timeEarly detectionVertebrate cellsArtificial cell constructsAvian leukosis virusesVirus
Owner:YANGZHOU UNIV
Pathogen containment device
PendingUS20210338361A1Breathing protectionTracheal tubesPathogenMycobacterium
Owner:MAJZOUB IBRAHIM +1
Rapid Enzyme-Linked Immunosorbant Assay for Detection and Identification of Pathogens and Determination of Antimicrobial Susceptibility
ActiveUS20140315219A1High sensitivityStrong specificityBioreactor/fermenter combinationsBiological substance pretreatmentsNon targetedNon target
Owner:BARNHIZER BRET T +1
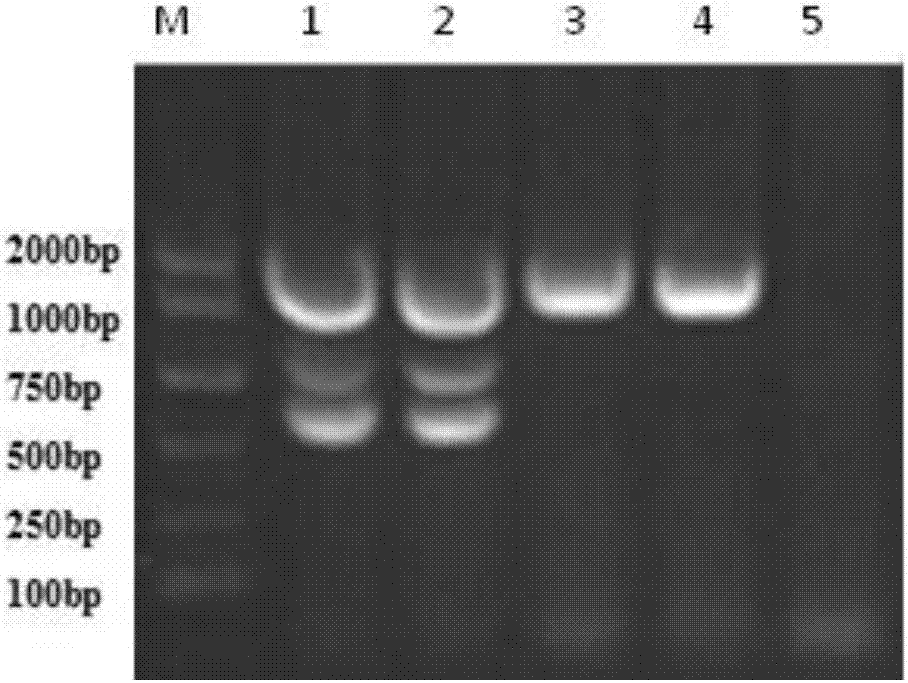
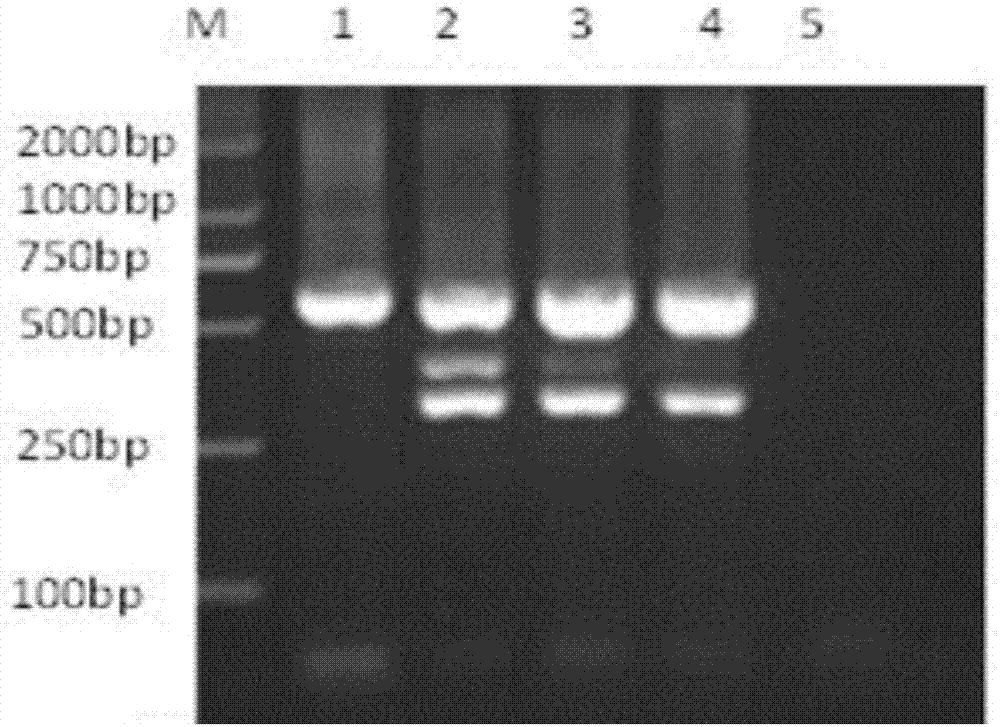
Multiple nested PCR (polymerase chain reaction) detection primer, kit and method for Neospora caninum, Brucella abortus and infectious bovine rhinotracheitis virus
InactiveCN107012260AMicrobiological testing/measurementMicroorganism based processesPathogenReal-time polymerase chain reaction
Owner:HENAN UNIV OF SCI & TECH
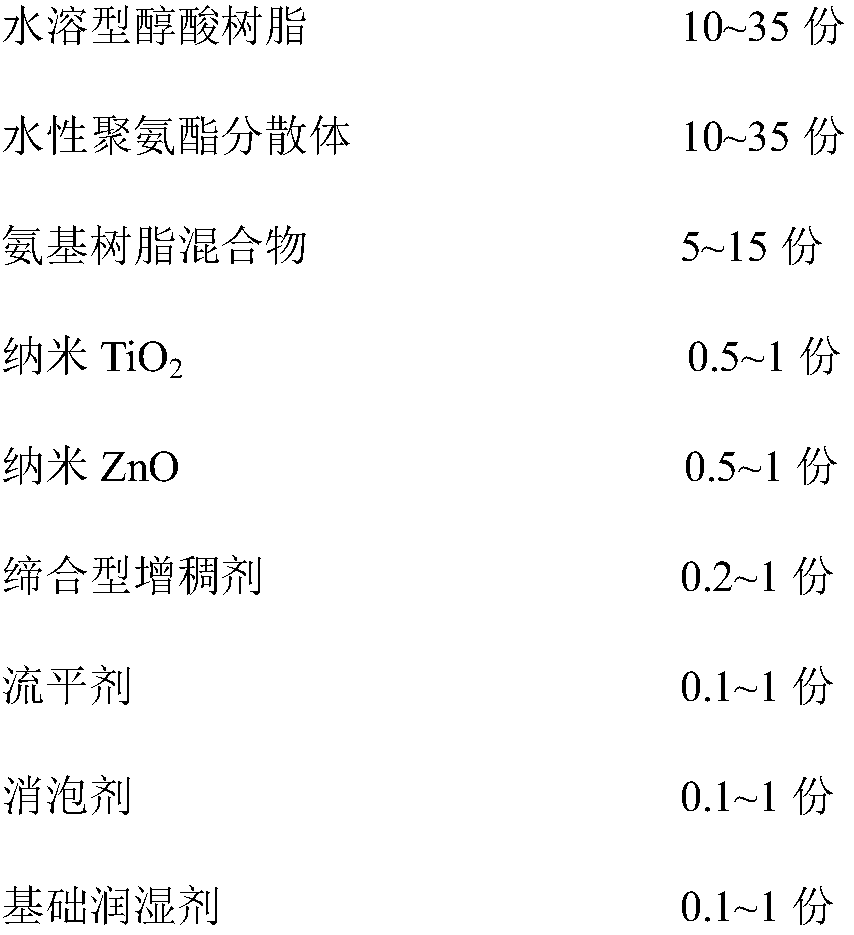
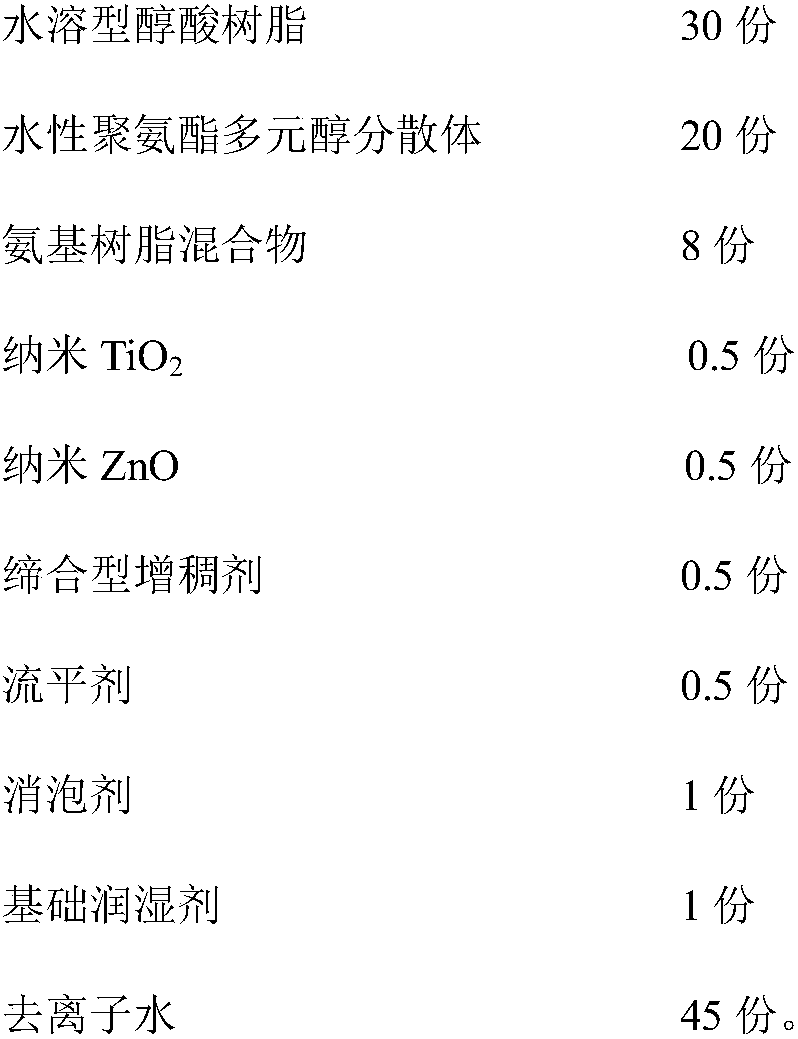
Durable antibacterial waterborne woodenware coating and preparation method thereof
ActiveCN108165146AImprove antibacterial propertiesOvercoming volatileAntifouling/underwater paintsPaints with biocidesAssociation typePolyamide
Owner:肇庆易涂宝涂料有限公司
Method for rapidly detecting pathogen of rice black-streaked dwarf
InactiveCN104450960AReduced Possibility of ContaminationImprove throughputMicrobiological testing/measurementSolubilityTotal rna
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Reagent kit for Coxsackie virus CA10 type fluorescent quantitative PCR detection
InactiveCN105331748AStrong specificityGood detection rateMicrobiological testing/measurementMicroorganism based processesCoxsackie VirusesRNA extraction
The embodiment of the invention discloses a reagent kit for Coxsackie virus CA10 type fluorescent quantitative PCR detection. The reagent kit comprises an RNA extraction solution, RNA eluant, an RT-PCR reinforcing agent, an internal label, a PCR reaction solution, a CA10 positive reference substance and a CA10 negative reference substance. By means of the reagent kit for Coxsackie virus CA10 type fluorescent quantitative PCR detection, only CA10-RNA can be detected, and other pathogene RNA can not be detected. Besides, detection sensitivity, accuracy and stability are high, detection sensitivity can reach 50 copie / ml, the detection range ranges from 5.00E+01 to 5.00E+09 copies / ml, and reliable experiment evidences are provided for early diagnosis of Coxsackie virus CA10 type infection.
Owner:SANSURE BIOTECH INC
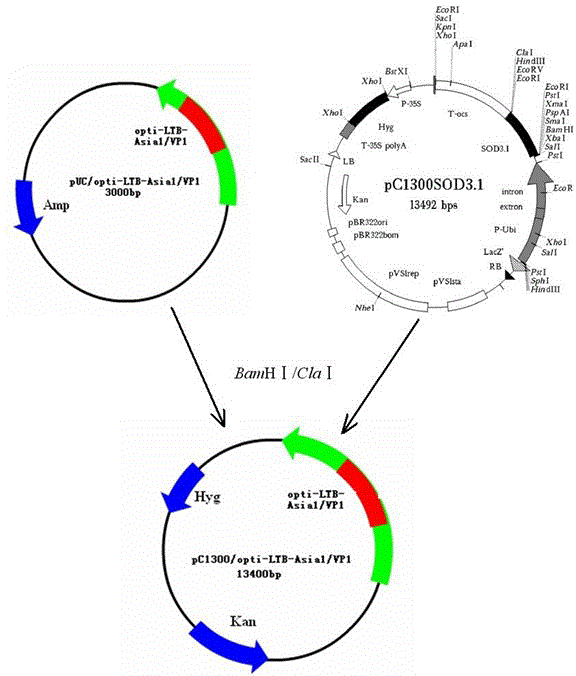
Asia 1 type foot and mouth disease virus antigen and preparation and application thereof
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for synchronously detecting five important virus pathogens in ranunculus asiaticus
ActiveCN112029907AQuick checkAccurate detectionMicrobiological testing/measurementMicroorganism based processesMultiplexTotal rna
Owner:云南省农业科学院生物技术与种质资源研究所
Method for detecting infectious pathogens and portable detector
InactiveCN101886118AEasy to carryStrong specificityMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementDiagnosis earlyUltraviolet lights
Owner:HAI KANG LIFE
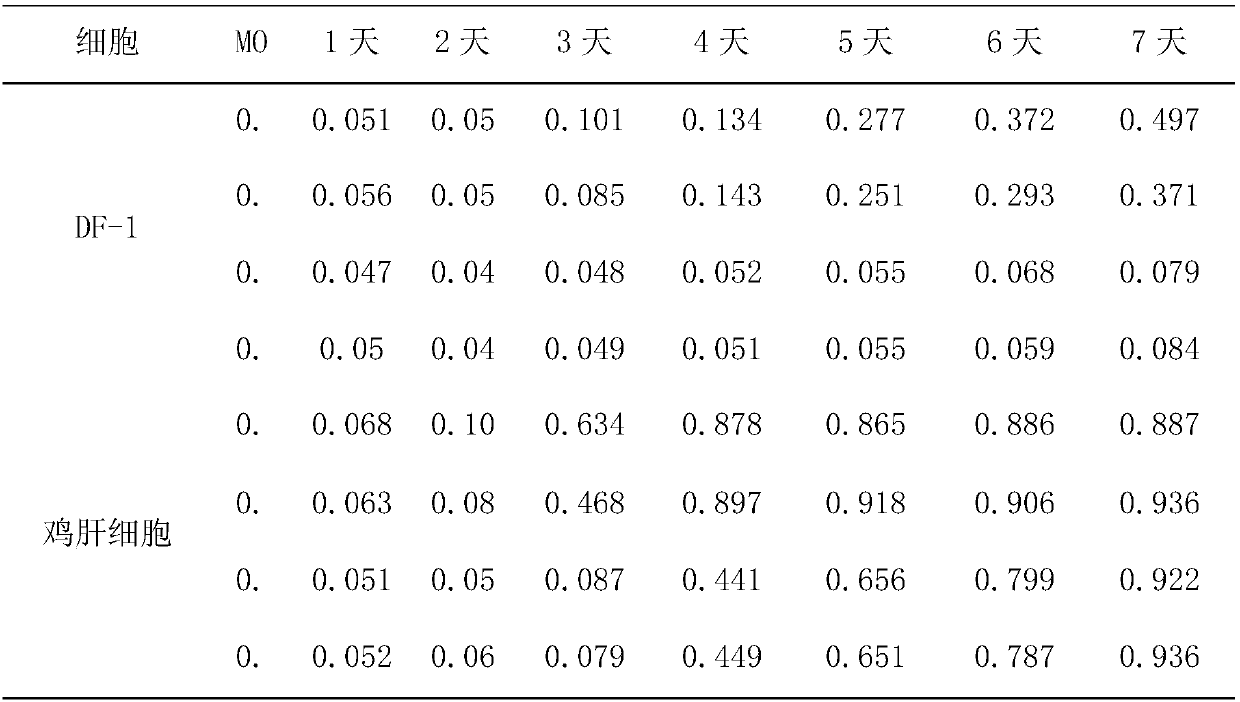
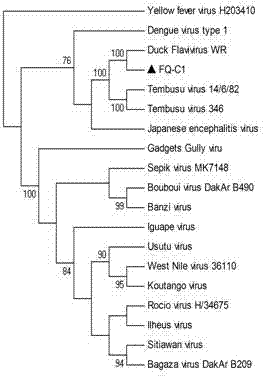
Chicken Flavivirus and inactivated vaccine thereof
ActiveCN102229916AGood antigenicityImprove securityViral antigen ingredientsMicroorganism based processesBiotechnologyInfectious Disorder
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap