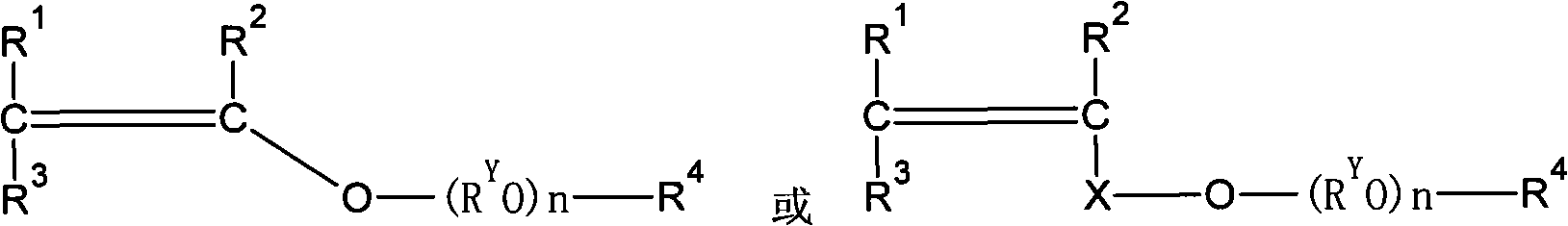
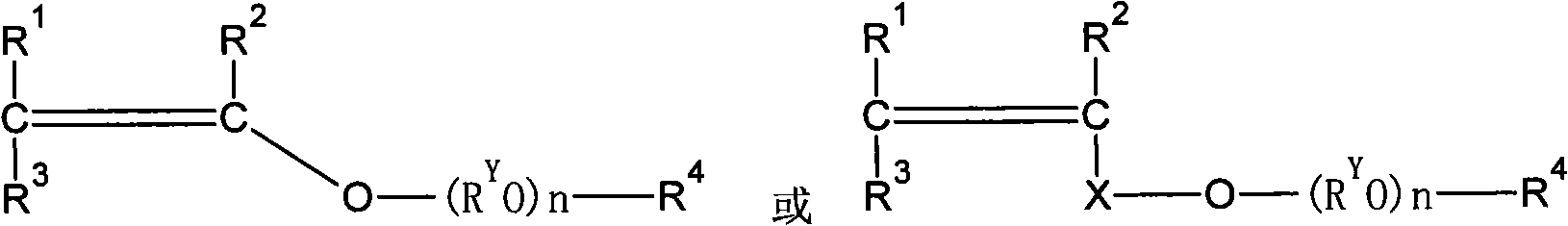
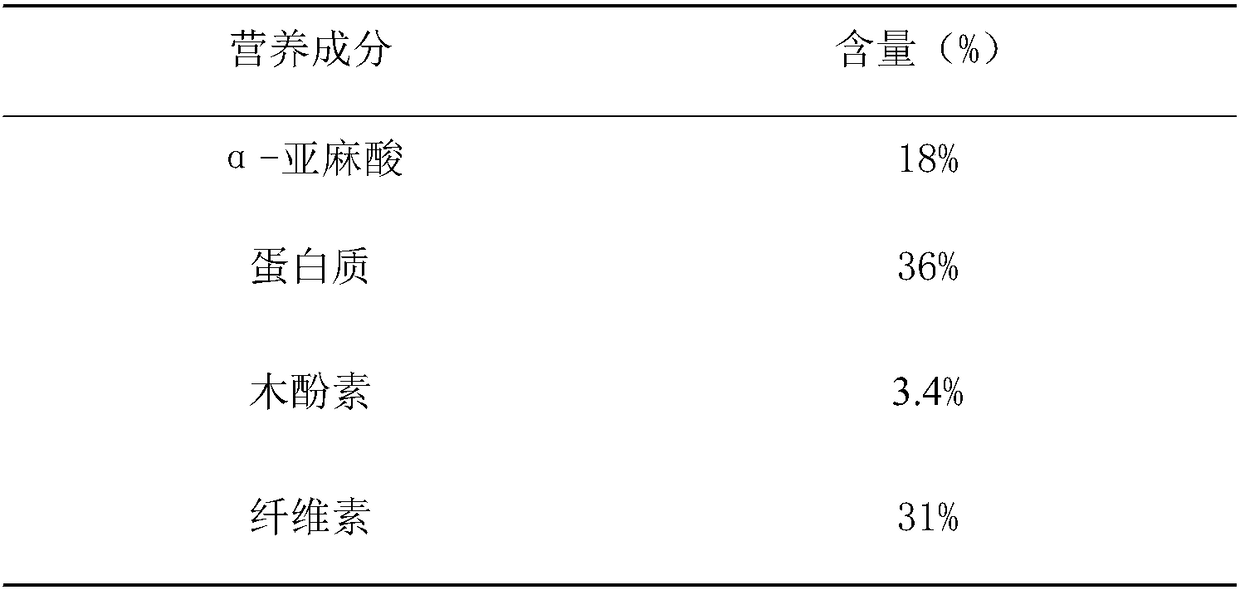
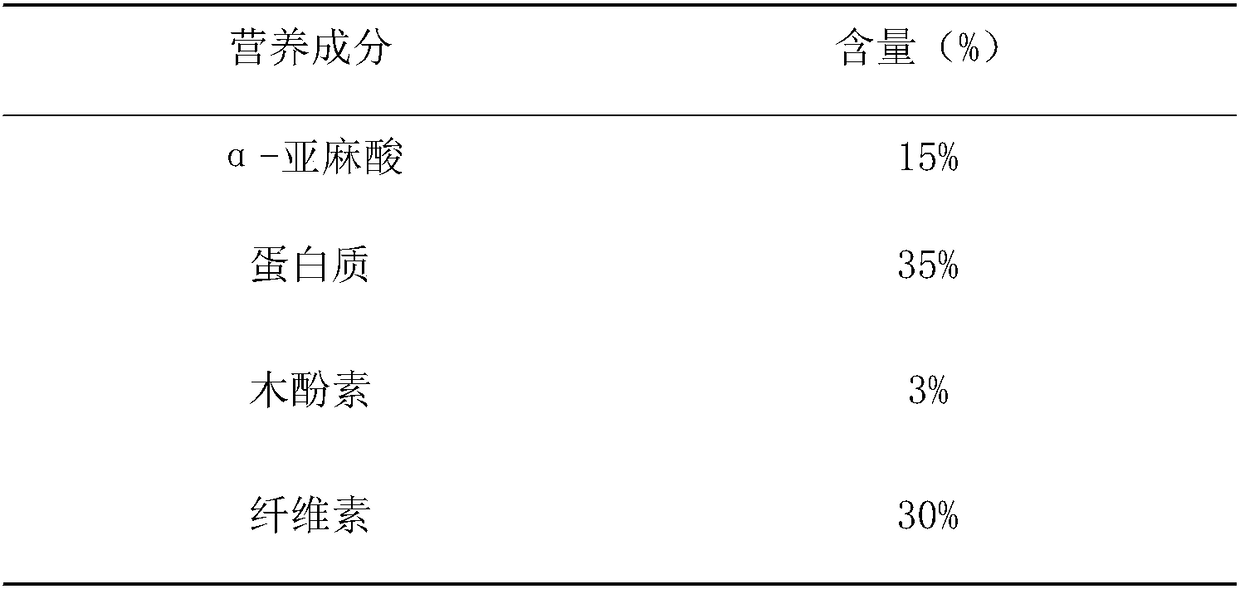
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
12 results about "Unsaturated fatty acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serum-free culture system for efficiently culturing human umbilical cord mesenchymal stem cells in vitro
InactiveCN104164405AGood repeatabilityImprove passaging stabilitySkeletal/connective tissue cellsAntioxidantCytokine
The invention relates to a serum-free culture system for efficiently culturing human umbilical cord mesenchymal stem cells in vitro. The serum-free culture system comprises the following components: a basal culture medium a-MEM, cell factors, hormones and proteins, unsaturated fatty acids, antioxidants, energy substances, an amino acid additive, vitamins and metal additives. The serum-free culture system comprises simple and clear components, is free from harms of pathogens, does not have difference between batches, has good repeatability, can obtain plenty of high-quality human umbilical cord mesenchymal stem cells in a short time and has high passage stability, so that the serum-free culture system can be applied to scientific researches and can provide high-purity vibrant cells for cell therapy as a mating system in cell therapy.
Owner:CYAGEN BIOSCI INC
Method of fast degradating pine dust rosin kind substance
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY +1
Method for extracting unsaturated fatty acids from eucalyptus leaves
InactiveCN102604735ALow costSimple extraction methodFatty-oils/fats refiningFatty-oils/fats productionCholesterolDissolution
Owner:CHINA EUCALYPT RES CENT
Method of preparing polyvinylchloride composite processing auxiliary agent
InactiveCN101016396AEthylene bisUnsaturated fatty acid
Owner:YIXING SANLIAN CHEM FACTORY
Preparation method of concrete water reducer
The invention provides a preparation method of a concrete water reducer, comprising the following steps: 1) letting MPEG and unsaturated acid be subject to esterification to obtain a mixture of MPEG unsaturated fatty acid and unsaturated acid (salt); 2) dissolving nonsaturated polyether; 3) adding an initiator, and adding the mixture of MPEG unsaturated fatty acid and unsaturated acid (salt), a chain transferring agent and a promoter dropwisely; and 4) keeping warm and aging, neutralizing with alkali, and discharging. By using a copolymerization method to copolymerize the ester unsaturated monomer and the ether unsaturated monomer, the water reducer with good adaptability is prepared.
Owner:SHANGHAI TAIJIE CHEM
Soft shell crab dry powder and processing method thereof
Owner:NINGBO UNIV
Ready-to-eat diet linseed meal and preparation method thereof
Owner:广州利众生物科技有限公司
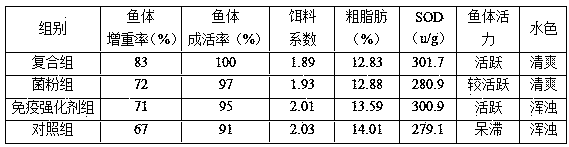
Compound preparation capable of effectively improving disease resistance of aquatic animals and preparation method thereof
InactiveCN108094686APromote digestion and absorptionEnhance non-specific immunityClimate change adaptationAnimal feeding stuffDiseaseFeces
Owner:江苏三仪生物工程有限公司 +1
Brewing method of agaricus bisporus and chenopodium quinata wine
InactiveCN107746765AImprove immunityEnhance hypoglycemiaMetabolism disorderMicroorganism based processesAdditive ingredientGenus Chenopodium
Owner:JIANGSU HENGSHUN VINEGAR IND
Anti-counterfeiting waterborne ink for medicine packaging and preparation method of anti-counterfeiting waterborne ink
ActiveCN106366766AExcellent printing suitabilityEasy to operateInksBiochemical engineeringUnsaturated fat
The invention discloses anti-counterfeiting waterborne ink for medicine packaging and a preparation method of the anti-counterfeiting waterborne ink. The anti-counterfeiting waterborne ink is prepared from the following components in parts by mass: 30 parts of water, 13 parts of polyurethane, 9 parts of a pigment, 32 parts of unsaturated fatty acid, 3 parts of a heat discoloration microcapsule, 12 parts of C12H22O11, 0.2 part of lecithin and 0.05 part of xanthan gum. The preparation method of the anti-counterfeiting waterborne ink for the medicine packaging is simple to operate, is safe and environmental friendly, lowers the production cost and is suitable for industrial production. The anti-counterfeiting waterborne ink prepared by adopting the method is excellent in printing property and is safe and non-toxic.
Owner:张文涛
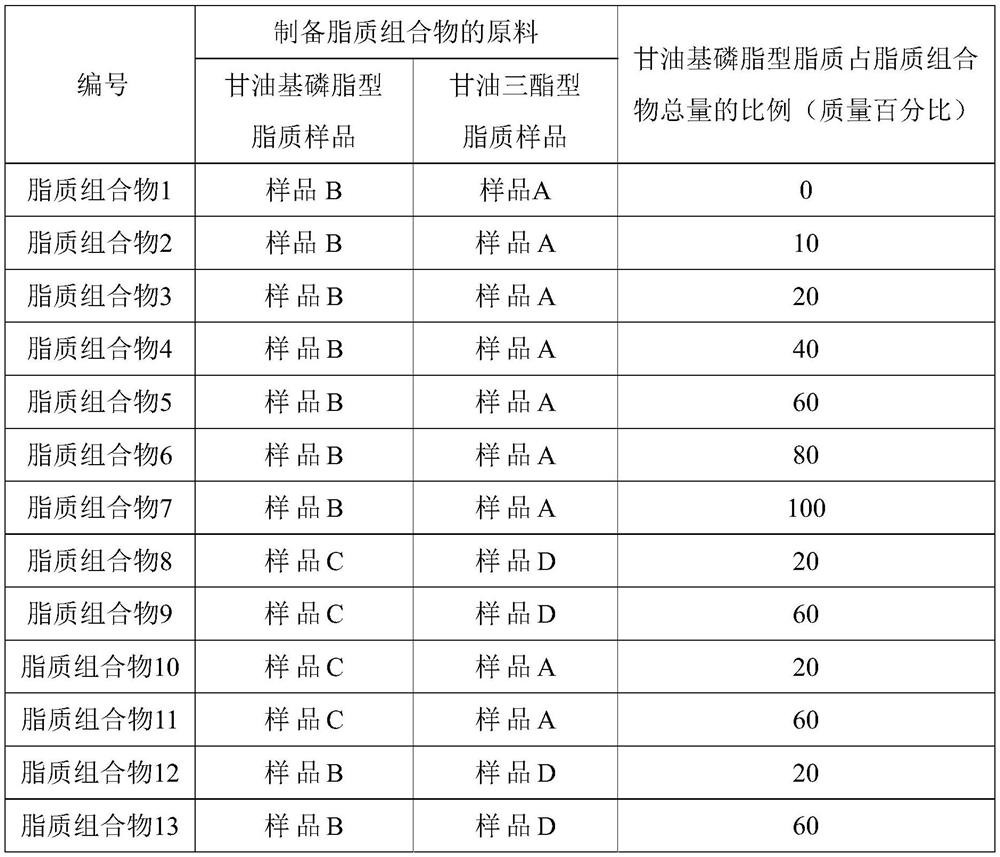
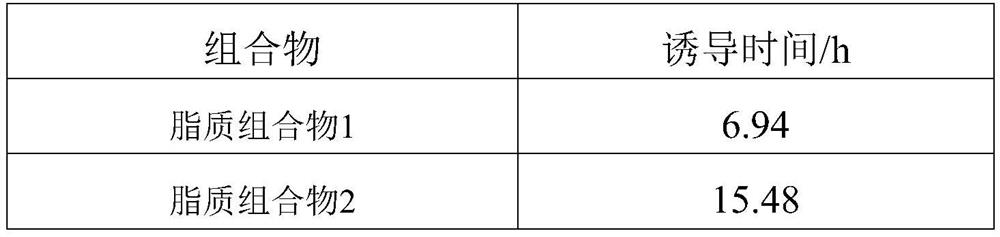
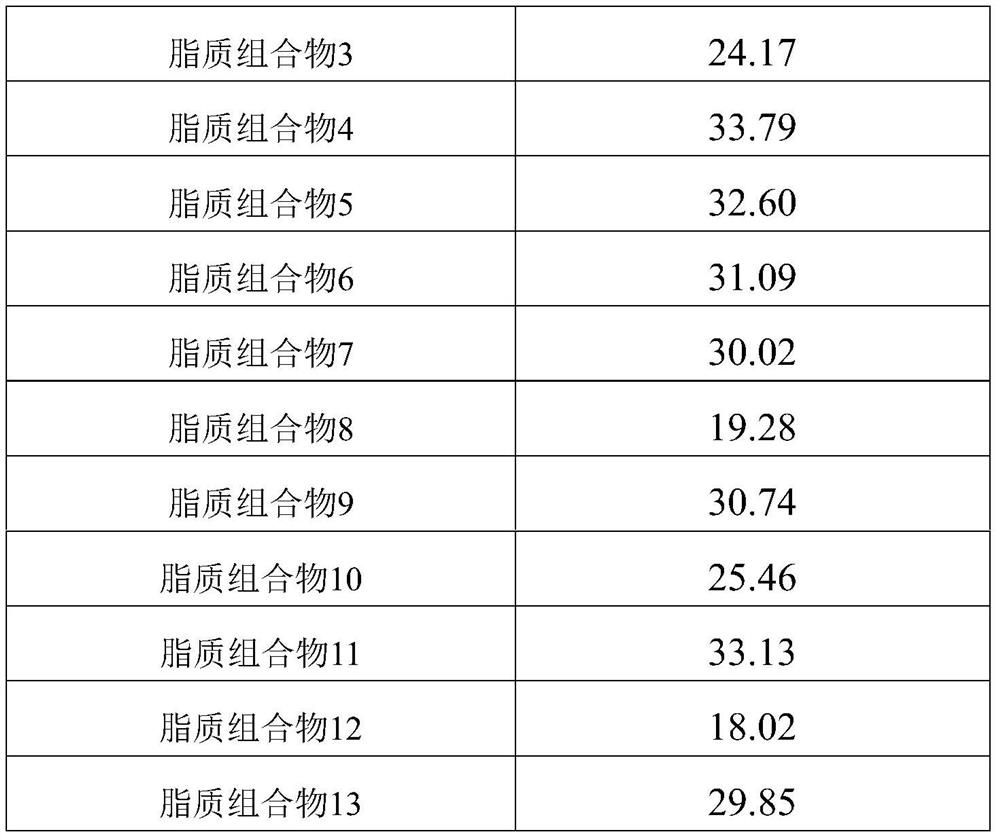
Lipid composition
Owner:蜜儿乐儿乳业(上海)有限公司 +1
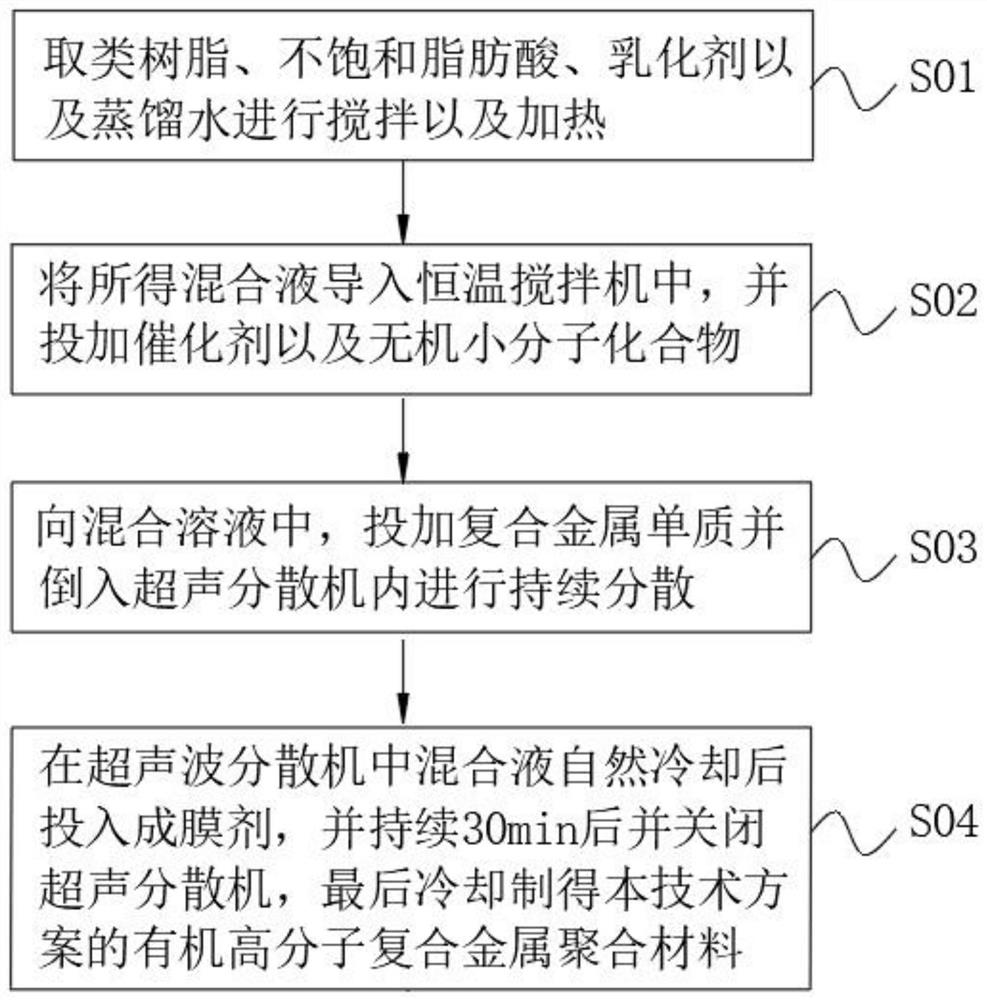
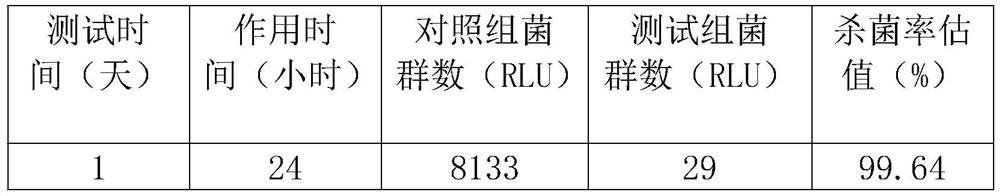
Organic polymer composite metal polymeric material
PendingCN113337209AAntifouling/underwater paintsPaints with biocidesUnsaturated fatty acidComposite material
Owner:贺征 +1
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap