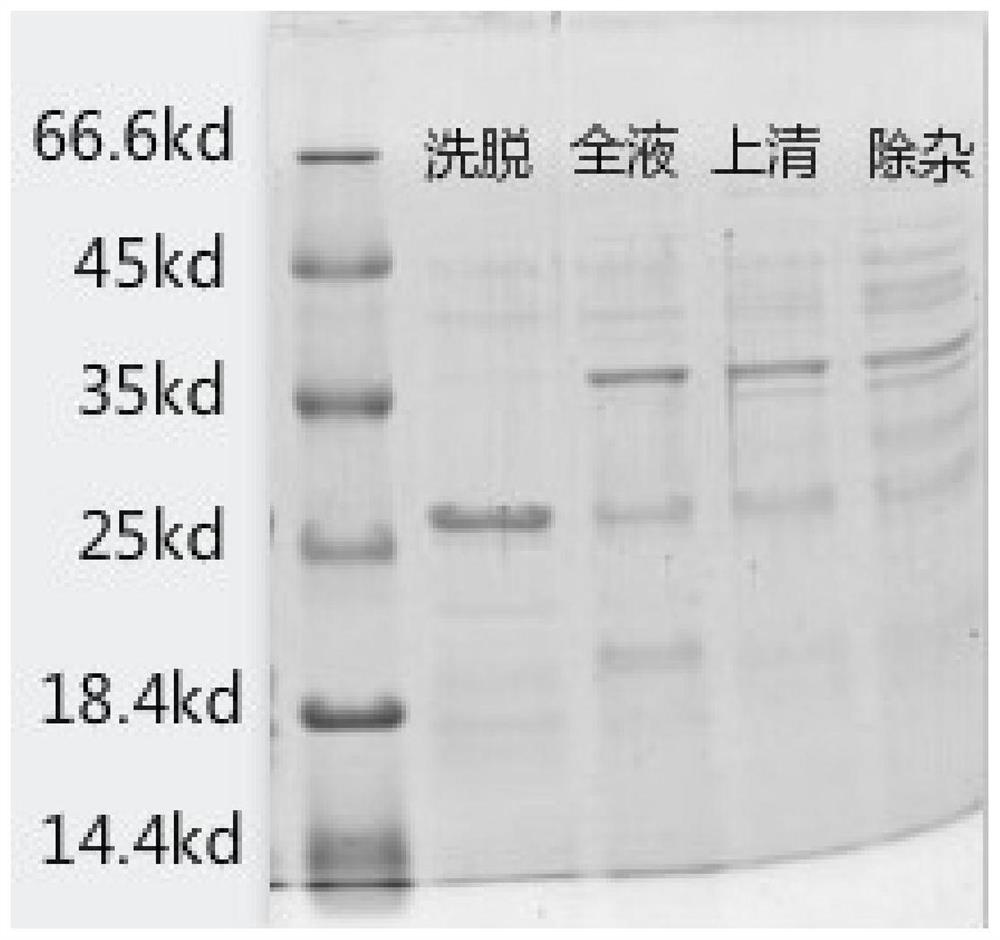
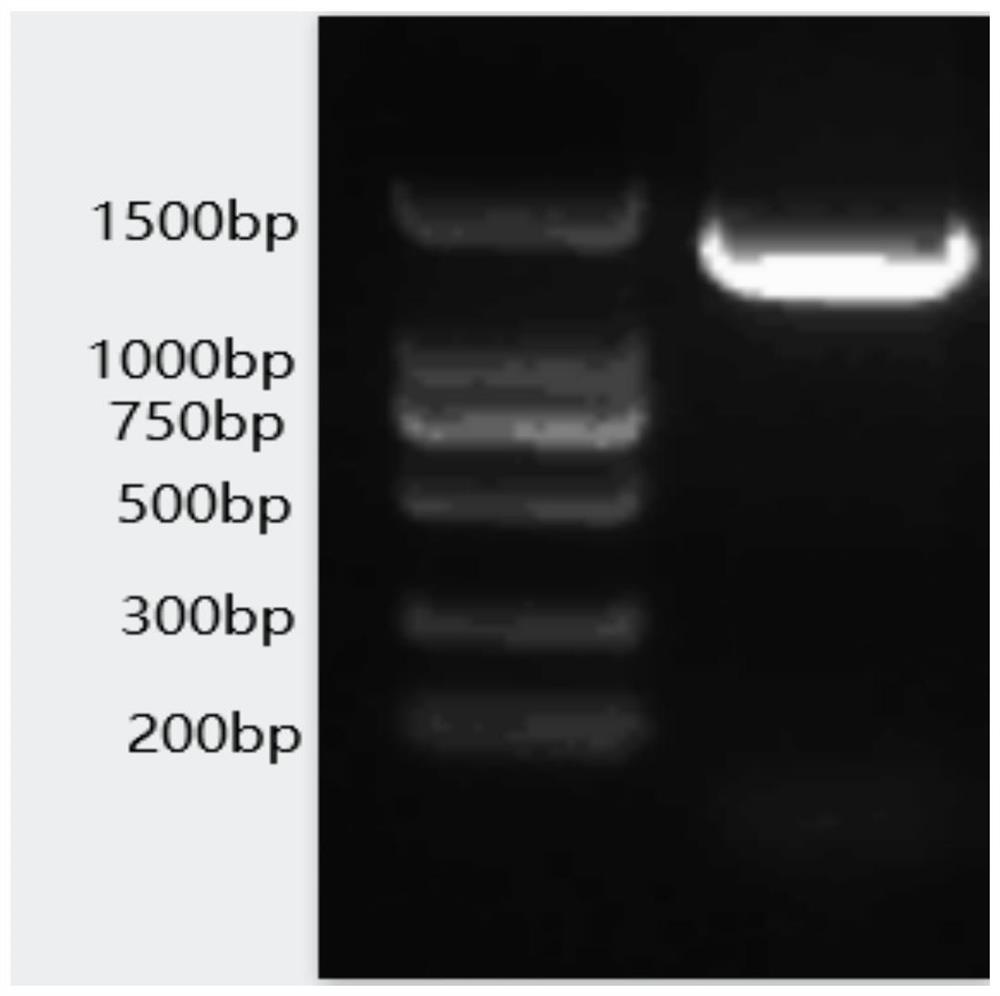
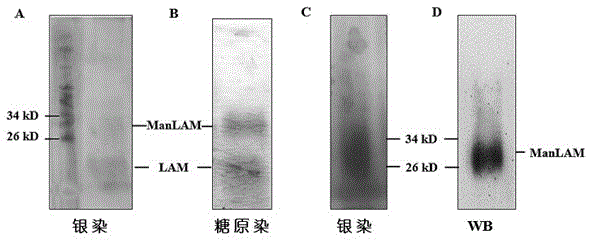
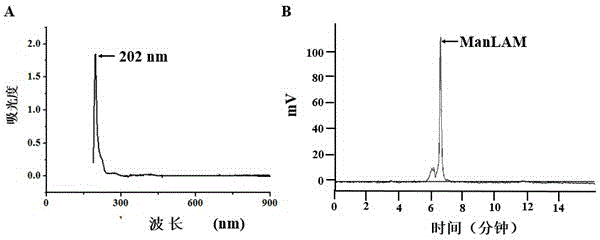
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
24 results about "Virology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virology is the study of viruses – submicroscopic, parasitic particles of genetic material contained in a protein coat – and virus-like agents. It focuses on the following aspects of viruses: their structure, classification and evolution, their ways to infect and exploit host cells for reproduction, their interaction with host organism physiology and immunity, the diseases they cause, the techniques to isolate and culture them, and their use in research and therapy. Virology is considered to be a subfield of microbiology or of medicine.
Method for treating melanoma using a herpes simplex virus and an immune checkpoint inhibitor
Owner:AMGEN INC
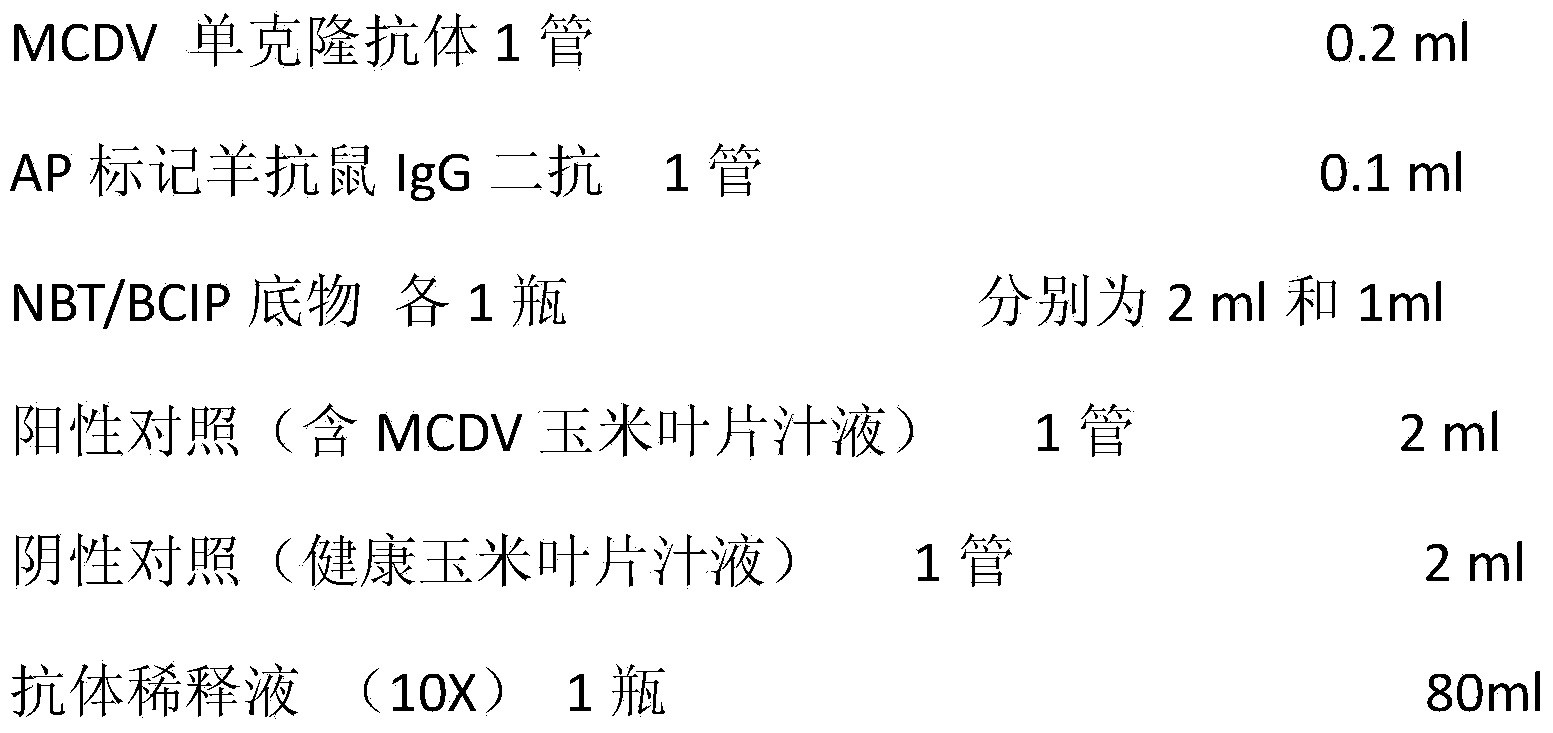
2019-nCoV double-target antibody detection microsphere complex combination, preparation method, kit and use method of kit
PendingCN111896734AReduce dosageEfficient detectionBiological material analysisViral testSpecific antibody
Owner:湖北新纵科病毒疾病工程技术有限公司
Dual fluorescent quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection kit for detecting PEDV-TGEV (Porcine Epidemic Diarrhea Virus-Transmissible Gastroenteritis Virus) nucleic acid
InactiveCN109097499AImprove throughputLow costMicrobiological testing/measurementMicroorganism based processesPositive controlFluorescence
The invention relates to the field of inspection and quarantine and aims to provide a dual fluorescent quantitative RT-PCR (Reverse Transcription-Polymerase Chain Reaction) detection kit for detectingPEDV-TGEV (Porcine Epidemic Diarrhea Virus-Transmissible Gastroenteritis Virus) nucleic acid. The kit comprises (1) an inverse transcription and fluorescent quantitative RT-PCR amplification mixed liquid, wherein the mixed liquid comprises an inverse transcription and fluorescent quantitative amplification primer and a probe and has sequence of SEQ ID NO: 1-6 as shown in the specification, and the inverse transcription primer is a 12 basic group random primer; (2) negative control; (3) positive control, wherein the positive control comprises positive control plasmid for the porcine epidemic diarrhea virus and the transmissible gastroenteritis virus. Compared with the prior art, the kit has the advantages of being high in flux, low in cost, good in specificity and high in sensitivity.
Owner:ZHEJIANG FORESTRY UNIVERSITY
One group of nucleic acid aptamers capable of specifically identifying Beijing genotypes tuberculosis bacterial strain antigen and application of nucleic acid aptamers
ActiveCN104450724AHigh affinityStrong specificityMaterial analysisDNA/RNA fragmentationAptamerAntigen
Owner:武汉顺可达生物科技有限公司
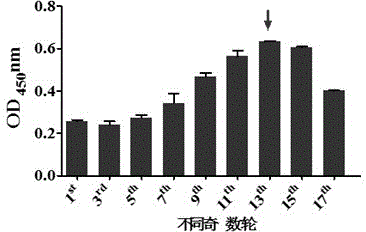
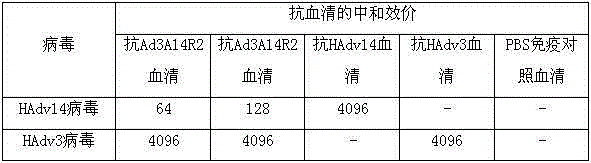
Application and preparation method of candidate strain of human type-3 adenovirus expressed human type-14 adenovirus neutralization epitope vaccine
InactiveCN105238766AAvoid infectionRetain major antigenic activityMicroorganism based processesAntiviralsAntigenHuman type
Owner:东莞市第八人民医院
Recombinant chimeric antigen of treponema pallidum as well as preparation method and use thereof
ActiveCN111575308AIncreased soluble expressionIncrease the range of applicabilityAntibody mimetics/scaffoldsBiological material analysisAntigenAntiendomysial antibodies
Owner:SICHUAN ANKERUI NEW MATERIAL TECH CO LTD
Soluble hiv-1 envelope glycoprotein trimers
ActiveUS20170035877A1Viral antigen ingredientsVirus peptidesHiv 1 vaccineHiv 1 envelope
The present application relates to novel HIV-1 envelope glycoproteins which may be utilized as an HIV-1 vaccine immunogens, antigens for crystallization and for the identification of broad neutralizing antibodies. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE +1
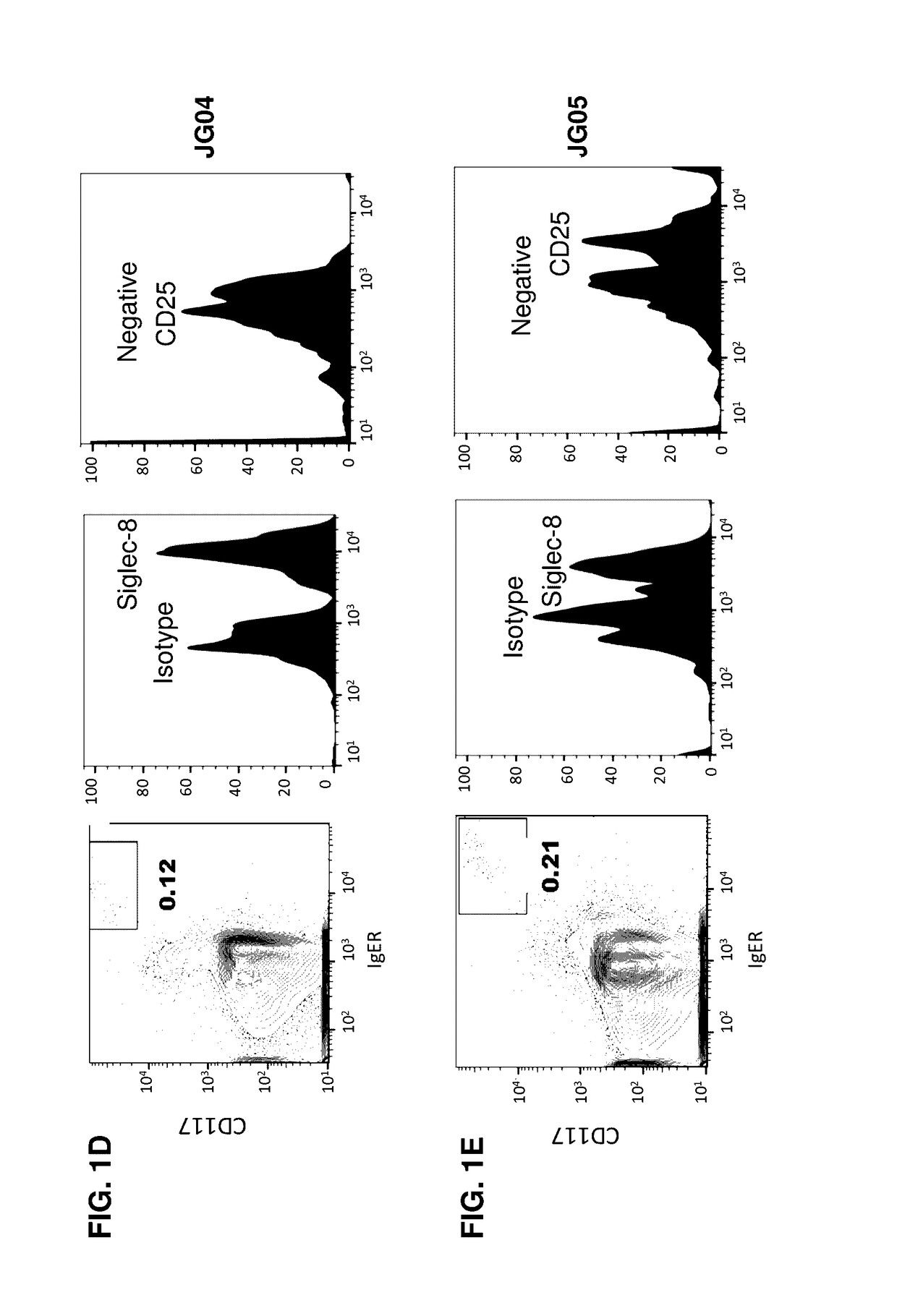
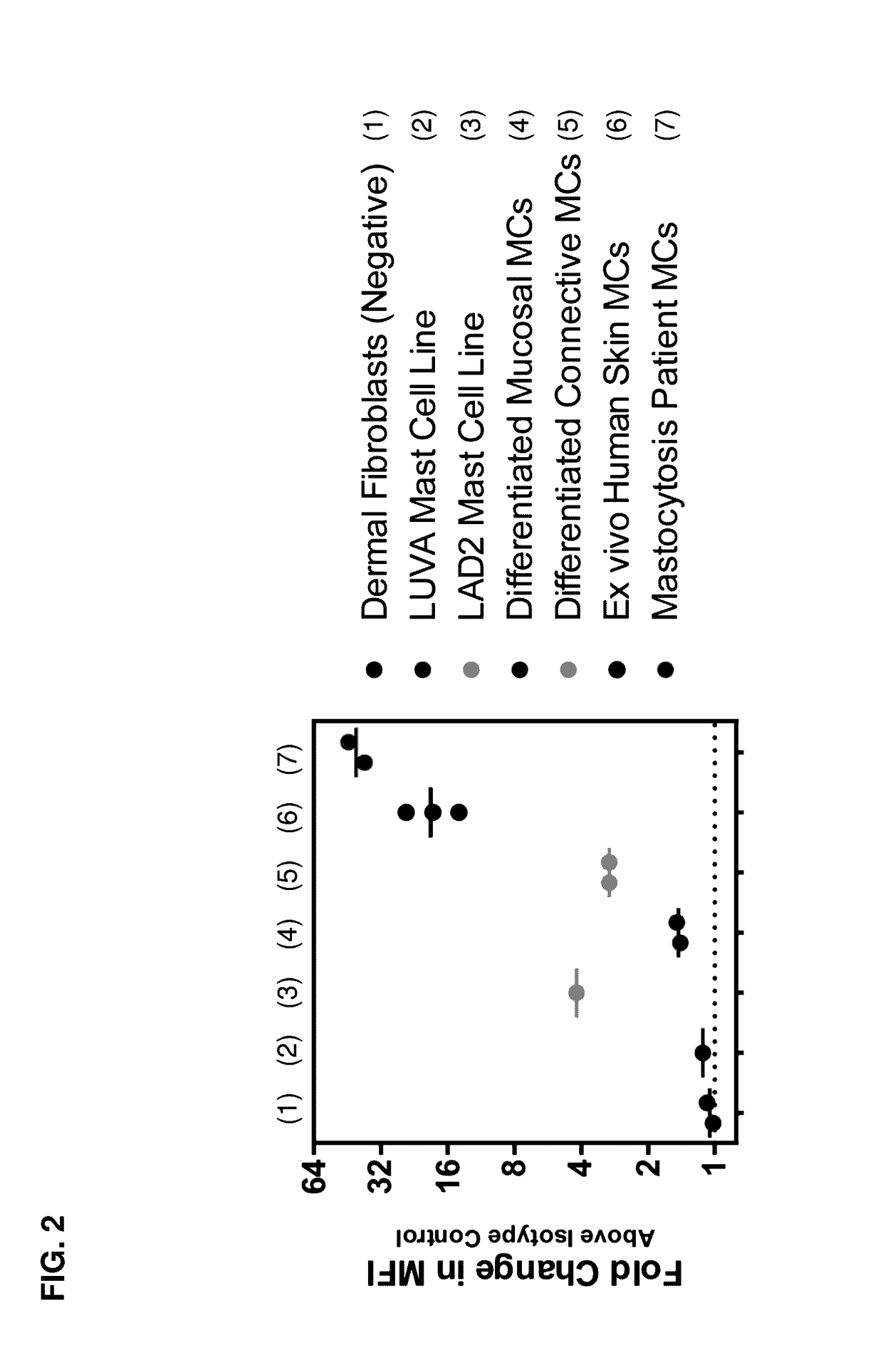
Methods and compositions for treating systemic mastocytosis
ActiveUS20170114138A1Improve antibody-dependent cell-mediated cytotoxicity (ADCC) activityEnhanced ADCC activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAgonist
Owner:ALLAKOS
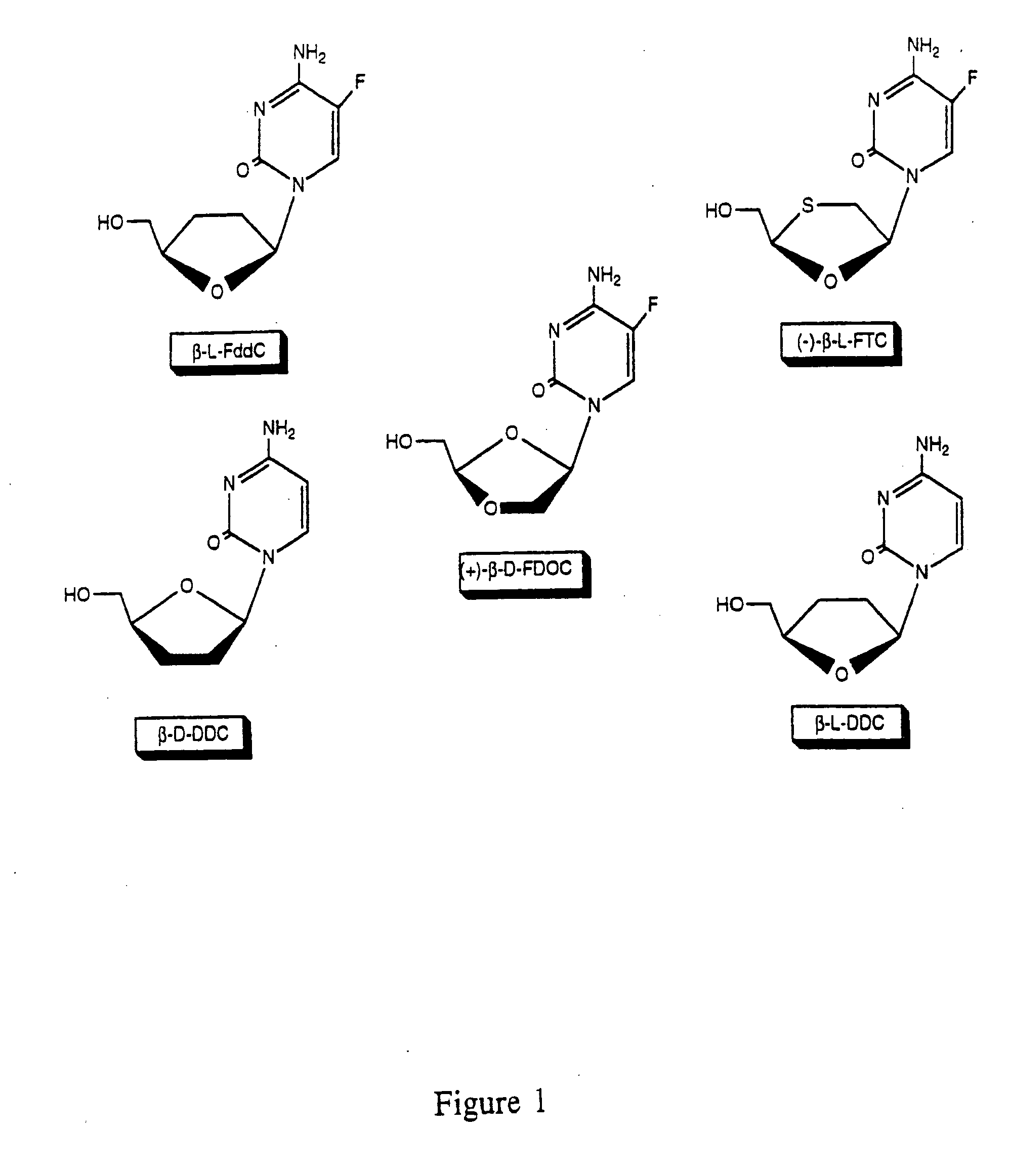
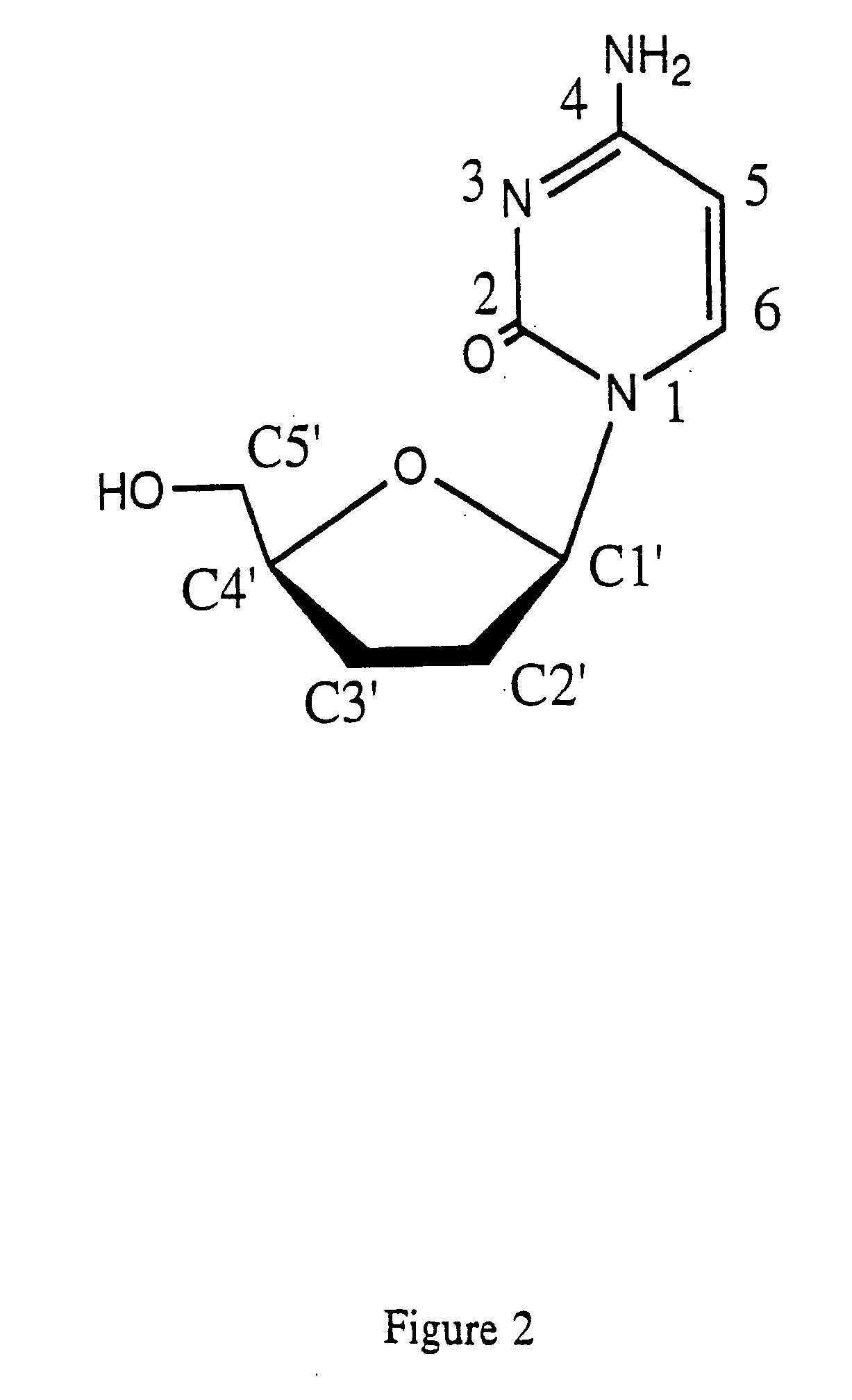
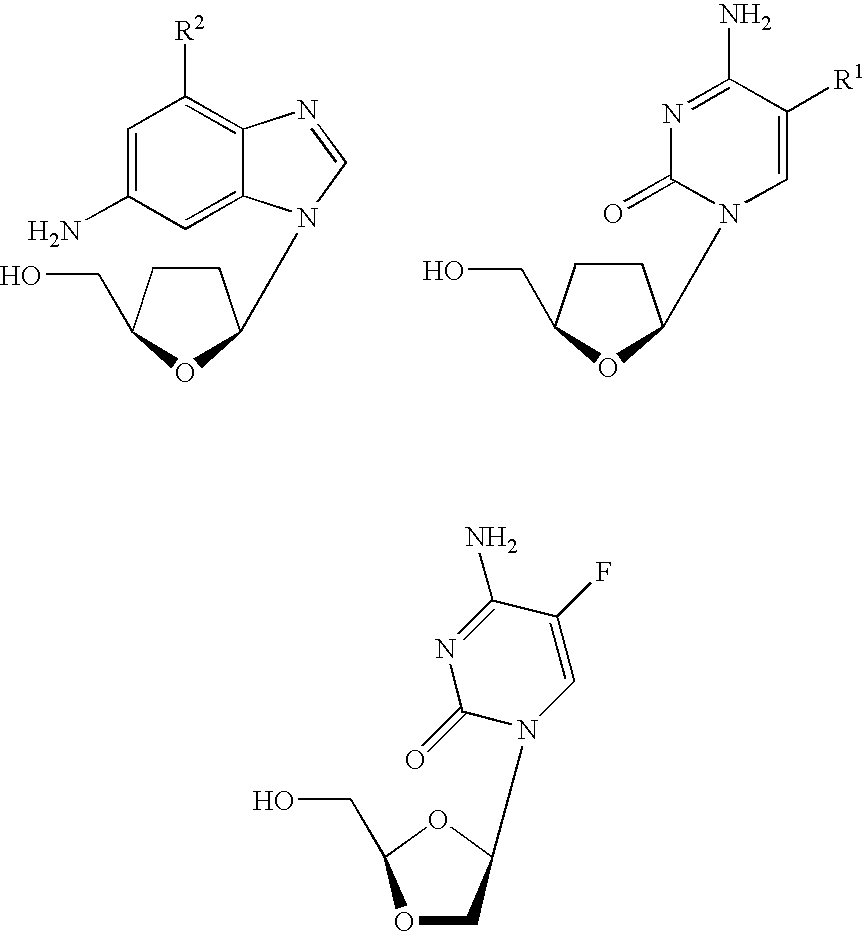
Thymine nucleosides with anti-hepatitis B virus activity
InactiveUS20050277616A1Prevent and retard progressionReduce usageBiocideSugar derivativesPhosphateThymine
Owner:OWENS HOWARD JR
Kit for detecting HSV-2 nucleic acid
InactiveCN109609699AStrong specificityGood repeatabilityMicrobiological testing/measurementMicroorganism based processesMinor grooveControl substances
The invention discloses a kit for detecting HSV-2 nucleic acid, comprising a specific primer pair HSV2-F and HSV2-R for detecting herpes simplex virus type 2, a fluorescent probe HSV2-P and a positivequality control substance. Both ends of the probe are modified separately, the 5' end is labeled with report fluorescein or fluorescent dye, and the 3' end is labeled with any minor groove binder without a fluorescent background. The positive quality control substance is a solution of a recombinant PUC57 plasmid containing a herpes simplex virus type 2 HSV-2 target gene gG sequence. The kit of the invention has the characteristics of fast and accurate detection, high resolution, high specificity, high efficiency and low cost, can realize qualitative detection of herpes simplex virus type 2 HSV-2, and has wide clinical application values.
Owner:ZHEJIANG PROVINCIAL PEOPLES HOSPITAL
Mycoplasma culture plate automatic interpreter
InactiveCN110669657AImprove efficiencyAvoid the problem of difficult to maintain consistent interpretation standardsBioreactor/fermenter combinationsBiological substance pretreatmentsMycoplasma cultureComputer printing
Owner:LIUZHOU CITY HEALTHCARE HOSPITAL FOR WOMEN & CHILDREN
Real-time fluorescent quantitative PCR (Polymerase Chain Reaction) detection primer for identifying FAdV-4 and DAdV-4 and kit thereof
InactiveCN112725532ASimplify operating proceduresLow costMicrobiological testing/measurementMicroorganism based processesFowl adenovirusVirology
The invention provides a real-time fluorescent quantitative PCR (polymerase chain reaction) detection primer for identifying fowl adenovirus type 4 (FAdV-4) and duck adenovirus type 4 (DAdV-4) and a kit thereof, and the sequence of the primer is shown as SEQ ID NO.1-2. A detection method established by utilizing the primer has very high specificity and sensitivity. At present, a primer research report of a real-time fluorescent quantitative PCR detection method capable of identifying two types of adenoviruses (FAdV-4 and DAdV-4) of adenovirus family aviadenoviruses infecting poultry by only one group of primers does not exist at home and abroad, and the establishment of the real-time fluorescent quantitative PCR detection method disclosed by the invention can fill up the blank of related fields at home and abroad.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Anti-cd19 humanized antibody and immune effector cell targeting cd19
ActiveUS20200062843A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesMurine antibody
Disclosed are an anti-CD19 humanized antibody prepared from a murine monoclonal antibody, a chimeric antigen receptor containing the humanized antibody, and an immune cell expressing the humanized antibody. Not only does the humanized antibody of the present invention not produce an anti-antibody response (AAR) and a human anti-mouse antibody response (HAMA), but same also has better affinity than a murine antibody, and has excellent activity and safety, thereby providing a new means for treating CD19-expressing tumors.
Owner:CRAGE MEDICAL CO LTD
Reagent kit for Coxsackie virus CA10 type fluorescent quantitative PCR detection
InactiveCN105331748AStrong specificityGood detection rateMicrobiological testing/measurementMicroorganism based processesCoxsackie VirusesRNA extraction
The embodiment of the invention discloses a reagent kit for Coxsackie virus CA10 type fluorescent quantitative PCR detection. The reagent kit comprises an RNA extraction solution, RNA eluant, an RT-PCR reinforcing agent, an internal label, a PCR reaction solution, a CA10 positive reference substance and a CA10 negative reference substance. By means of the reagent kit for Coxsackie virus CA10 type fluorescent quantitative PCR detection, only CA10-RNA can be detected, and other pathogene RNA can not be detected. Besides, detection sensitivity, accuracy and stability are high, detection sensitivity can reach 50 copie / ml, the detection range ranges from 5.00E+01 to 5.00E+09 copies / ml, and reliable experiment evidences are provided for early diagnosis of Coxsackie virus CA10 type infection.
Owner:SANSURE BIOTECH INC
Diagnostic reagent
PendingUS20220252595A1High sensitivityHigh strengthMaterial analysisAntigenTuberculosis mycobacterium
The invention provides a Mycobacterium Tuberculosis Complex (MTC) (for example, M. bovis and / or M. tuberculosis) diagnostic reagent comprising the reagent components:a. a Rv3616c antigen polypeptide and / or a Rv3616c antigenic cocktail;b. a Rv1789 antigen polypeptide and / or a Rv1789 antigenic cocktail;c. a Rv3810 antigen polypeptide and / or a Rv3810 antigenic cocktail; andd. a Rv3478 antigen polypeptide and / or a Rv3478 antigenic cocktail.The diagnostic reagent may further comprise one or more of a ESAT-6 antigen polypeptide and / or a ESAT-6 antigenic cocktail; a CFP-10 antigen polypeptide and / or a CFP-10 antigenic cocktail; a Rv3615c antigen polypeptide and / or a Rv3615c antigenic cocktail; and / or SEQ ID NO:207 (a fusion protein of ESAT-6 and CFP-10 and Rv3615c). There are also provided methods and kits involving use of the diagnostic reagent.
Owner:THE UK SEC FOR ENVIRONMENT
Primer combination and kit for identifying African swine fever virus gene deletion strain and African swine fever epidemic strain through centrifugal microfluidic chip
InactiveCN113215311AStrong specificityMake up detectionMicrobiological testing/measurementDNA/RNA fragmentationAfrican swine feverEpidemiology
Owner:SOUTH CHINA AGRI UNIV
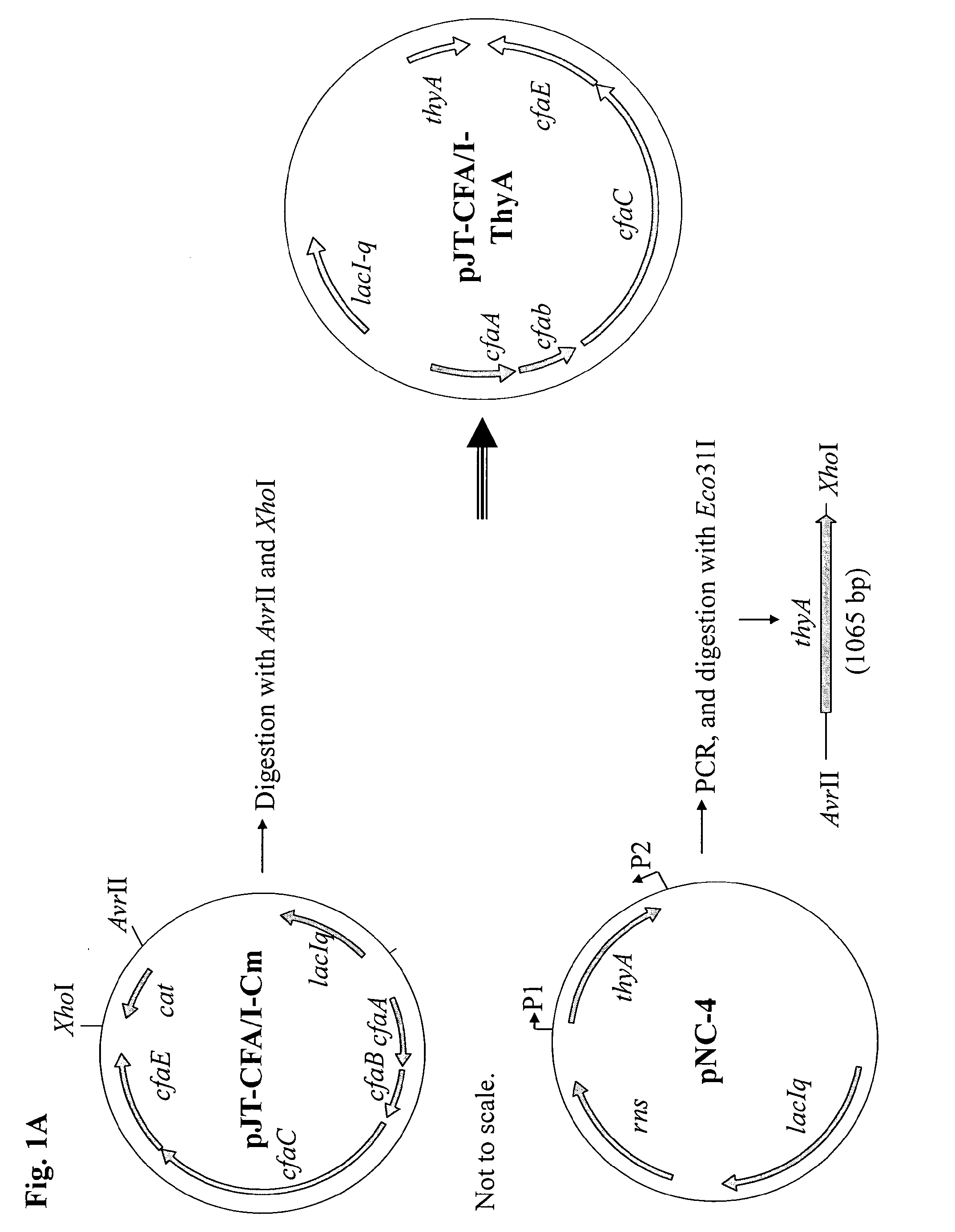
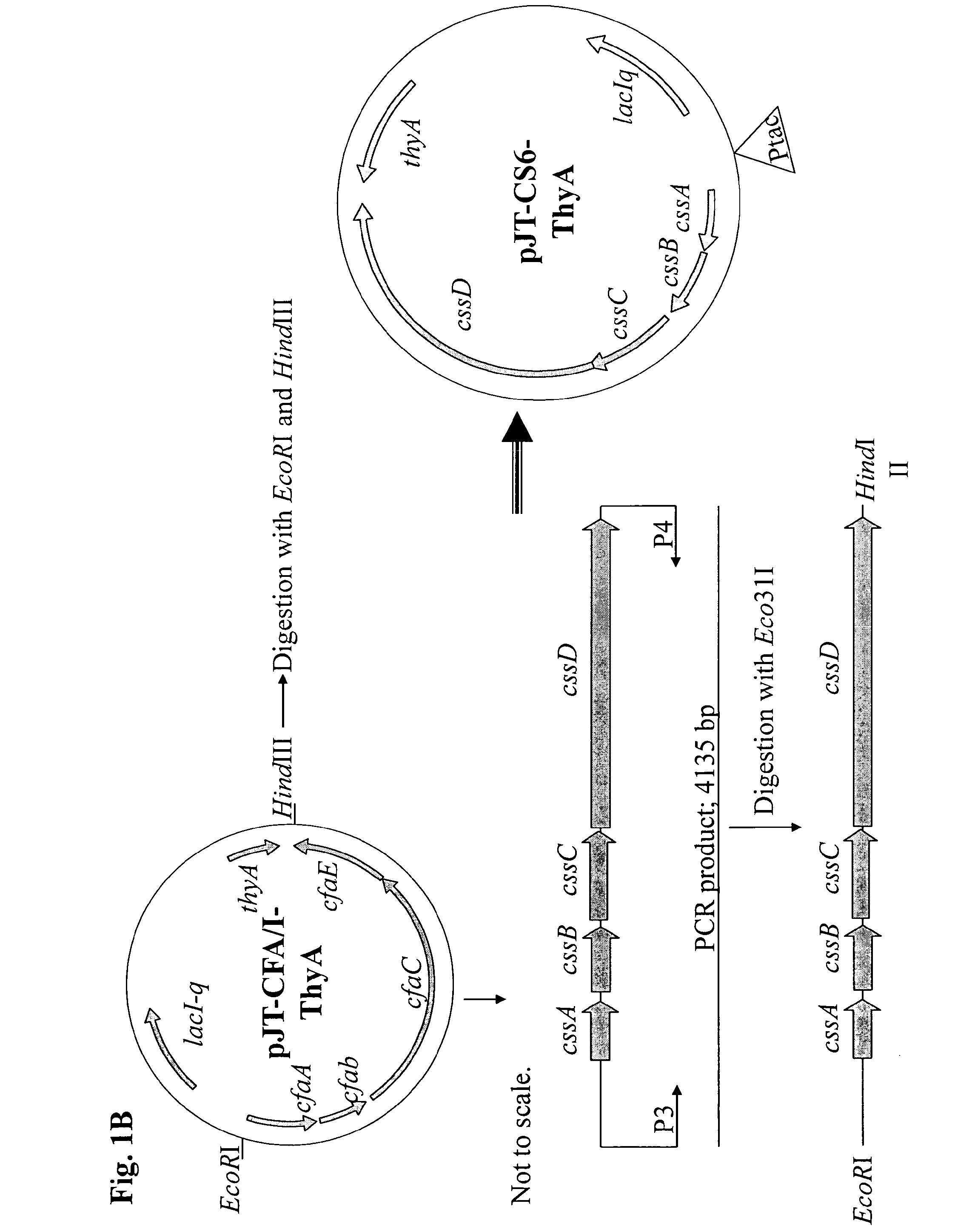
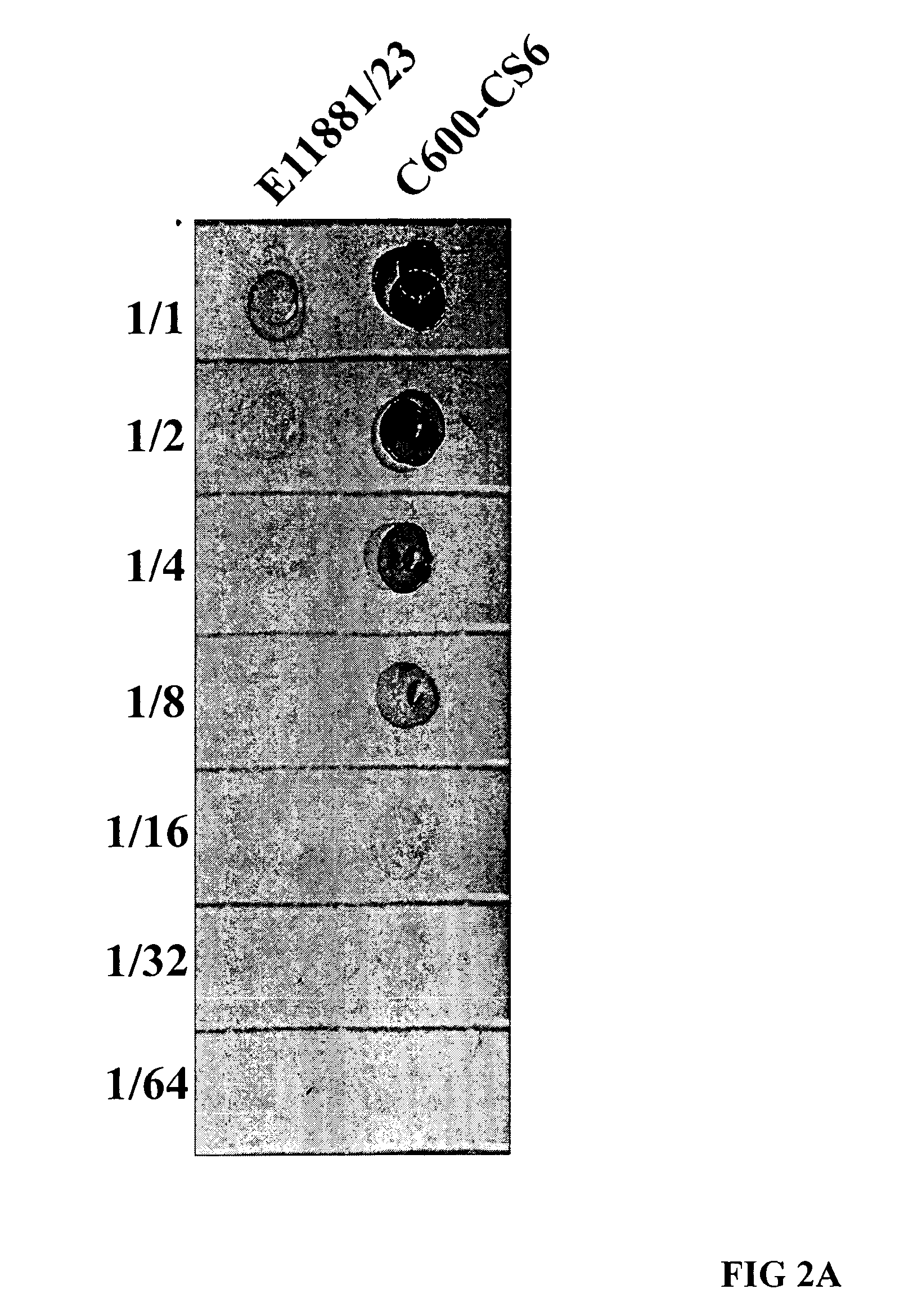
Method for increasing etec cs6 antigen presentation on cell surface and products obtainable thereof
Owner:SCANDINAVIAN BIOPHARMA HLDG
Phagemid vector construction method for constructing phage antibody library and antibody screening method
PendingCN112226417AEfficient screeningReduce screening roundsImmunoglobulins against cell receptors/antigens/surface-determinantsNucleic acid vectorPhage antibodiesCoat protein
Owner:SHANGHAI JIAO TONG UNIV
Hybridoma cell strain secreting anti-maize chlorotic dwarf virus monoclonal antibody, secreted antibody, application and kit prepared from monoclonal antibody
InactiveCN104328089AAccurate detectionSensitive detectionMicroorganism based processesImmunoglobulins against virusesCell strainHybridoma cell
Owner:QINGDAO ENTRY EXIT INSPECTION & QUARANTINE BUREAU P R CHINA
Method for detecting mycoplasma synoviae and reagent used in method
PendingCN114703302ALow dispersionLow negative fluorescence amplitudeMicrobiological testing/measurementMicroorganism based processesMycoplasma synoviaeGenomic Segment
The invention discloses a method for detecting mycoplasma synoviae and a reagent used by the method, and belongs to the technical field of nucleic acid or microorganism determination or detection methods. The invention discloses a method for detecting or assisting in detecting mycoplasma synoviae, which comprises the following steps of: performing digital PCR (Polymerase Chain Reaction) on a sample to be detected by utilizing a reagent or a kit, and determining whether the sample to be detected is the mycoplasma synoviae or contains the mycoplasma synoviae or is infected with the mycoplasma synoviae or not according to a digital PCR product, so as to determine whether the sample to be detected is the mycoplasma synoviae or contains the mycoplasma synoviae or is infected with the mycoplasma synoviae or is infected with the mycoplasma synoviae or is infected with the mycoplasma synoviae. The reagent or the kit comprises a chicken mycoplasma synoviae primer probe, and the chicken mycoplasma synoviae primer probe is composed of a primer and a probe, wherein the primer is used for specifically amplifying a chicken mycoplasma synoviae genome DNA fragment containing a DNA fragment with the nucleotide sequence of SEQ ID No.1, and the probe is specifically combined with the DNA fragment with the nucleotide sequence of SEQ ID No.1. The method can be used for detecting environmental samples and food products.
Owner:GUANGXI VETERINARY RES INST
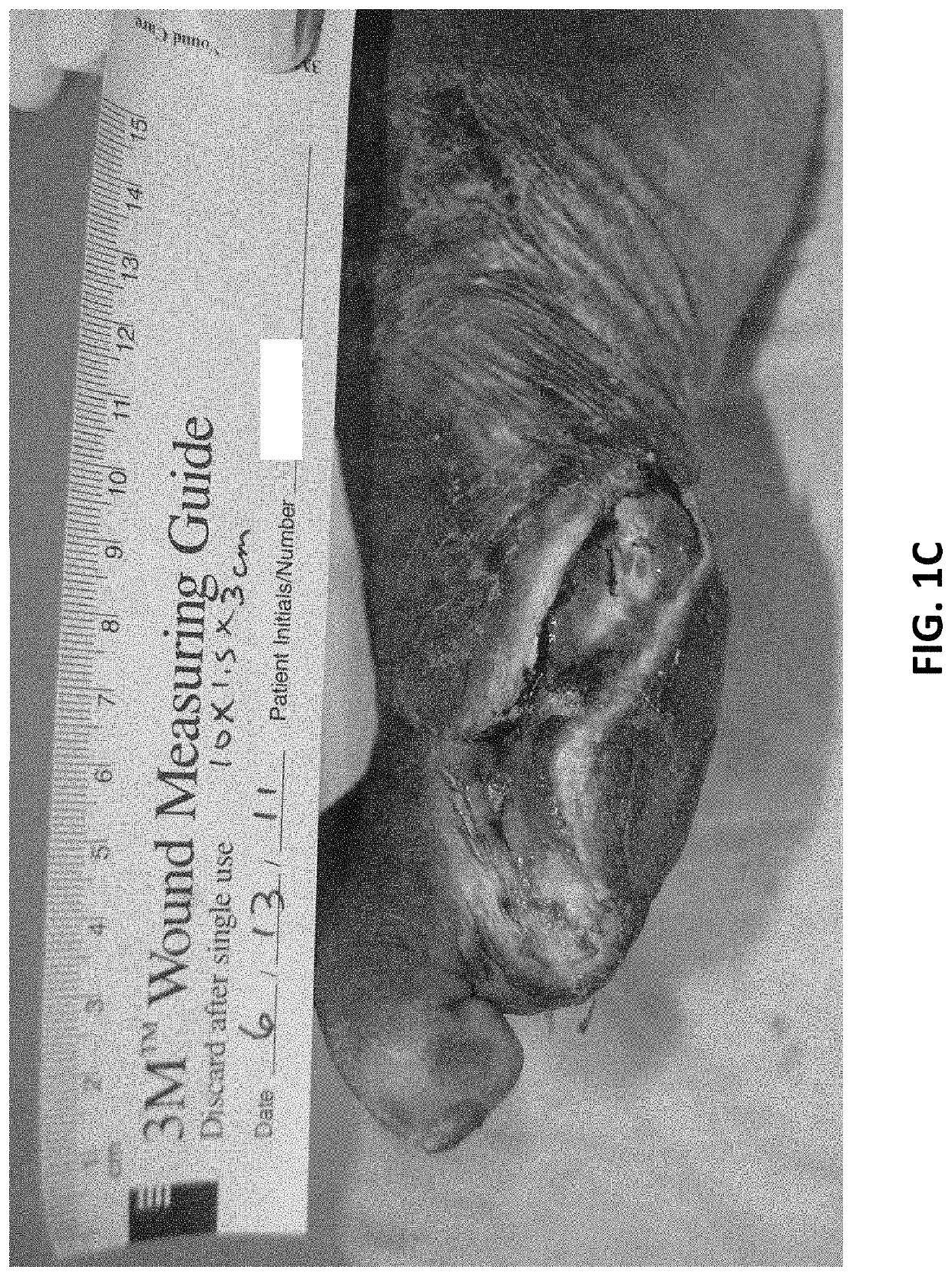
Formulations and methods for wound treatment
ActiveUS10905729B1Chronic woundOrganic active ingredientsPharmaceutical delivery mechanismBiotechnologyTopical treatment
Owner:SORUSH
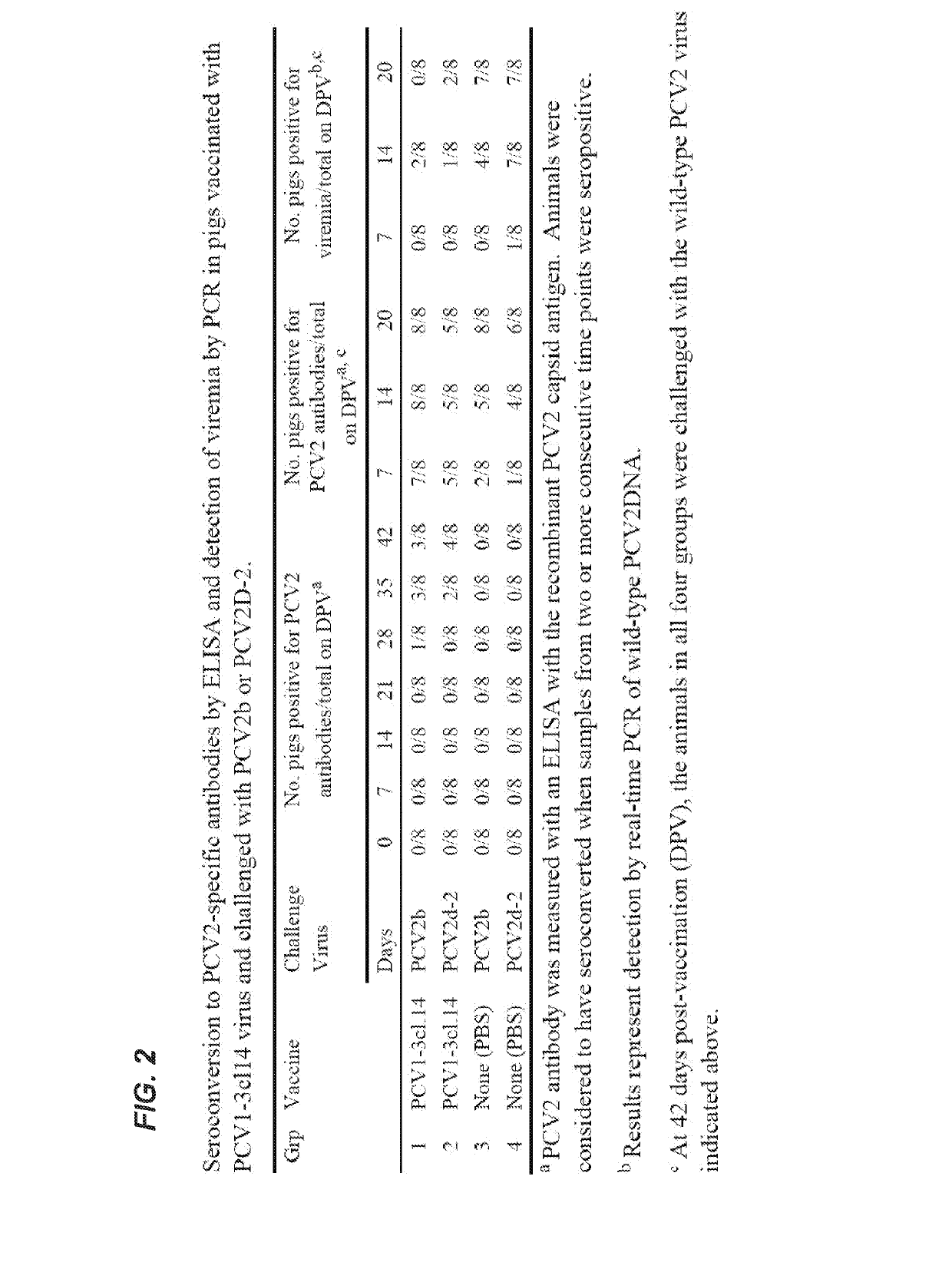
Chimeric porcine circovirus type 2 (PCV2) vaccines
InactiveUS20190091320A1Viral antigen ingredientsPharmaceutical delivery mechanismEpitopePorcine circovirus type 1
Owner:VIRGINIA TECH INTPROP INC
Recombinant baculovirus for expressing spike protein of chicken infectious bronchitis virus and application thereof
ActiveCN101948814AAvoiding the dangers of loose poisonsSuitable for useViruses/bacteriophagesFermentationAntigenShuttle vector
The invention relate to a recombinant baculovirus for expressing spike protein of chicken infectious bronchitis virus and application thereof, belonging to the biotechnical field. The recombinant baculovirus is prepared by a method comprising the following steps: obtaining S genes; building and indentifying a recombinant transfer vector pFastBacI-S; building and indentifying a recombinant shuttle vector AcIBV-SBacmid which infects Sf9 inset cells; and obtaining the recombinant baculovirus vAcIBV-S for expressing the spike protein of the chicken infectious bronchitis virus. The invention further provides the application of the recombinant baculovirus in the aspect of detecting chicken infectious bronchitis virus antibodies. The recombinant baculovirus can be used for detecting the chicken infectious bronchitis virus antibodies, thereby avoiding the risk of easy toxin diffusion when live virus serves as an antigen. The recombinant baculovirus can be used for detecting the chicken infectious bronchitis virus antibodies, can be used for quarantine on a large scale, and is especially suitable for use of quarantine departments at the basic level.
Owner:NAT VETERINARY BIOLOGICAL PROD ENG TECH RES CENT +1
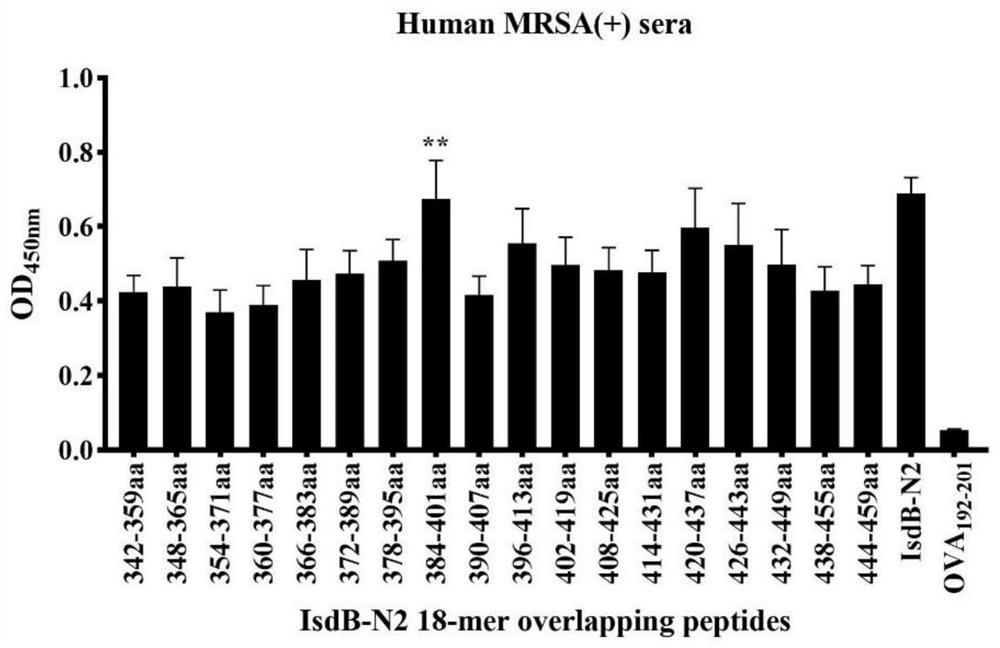
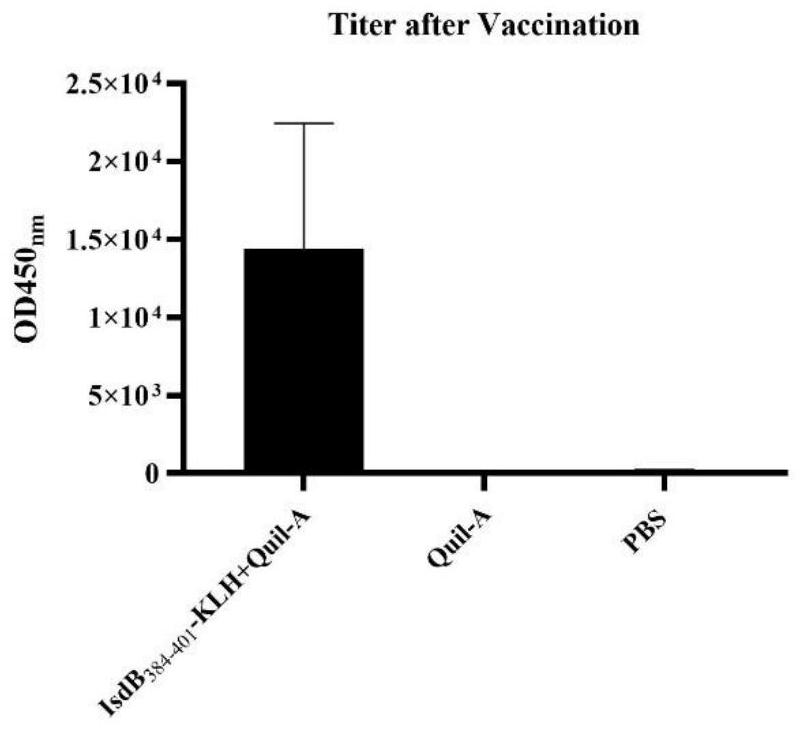
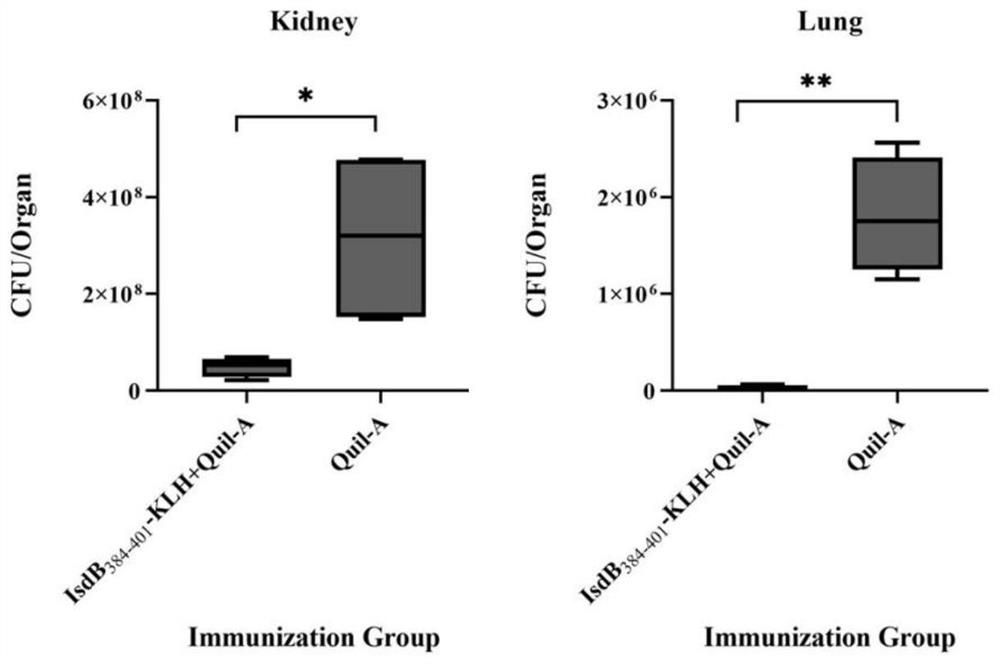
IsdB antigen epitope peptide for diagnosing or preventing staphylococcus aureus infection and application thereof
PendingCN112920259AImprove securityEfficient infectionConnective tissue peptidesAntibacterial agentsAntigen epitopeStaphyloccocus aureus
Owner:ARMY MEDICAL UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap