Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
24 results about "Blood plasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Blood plasma is a yellowish liquid component of blood that holds the blood cells in whole blood in suspension. It is the liquid part of the blood that carries cells and proteins throughout the body. It makes up about 55% of the body's total blood volume. It is the intravascular fluid part of extracellular fluid (all body fluid outside cells). It is mostly water (up to 95% by volume), and contains dissolved proteins (6–8%) (e.g. serum albumins, globulins, and fibrinogen), glucose, clotting factors, electrolytes (Na⁺, Ca²⁺, Mg²⁺, HCO₃⁻, Cl⁻, etc.), hormones, carbon dioxide (plasma being the main medium for excretory product transportation) and oxygen. It plays a vital role in an intravascular osmotic effect that keeps electrolyte concentration balanced and protects the body from infection and other blood disorders.
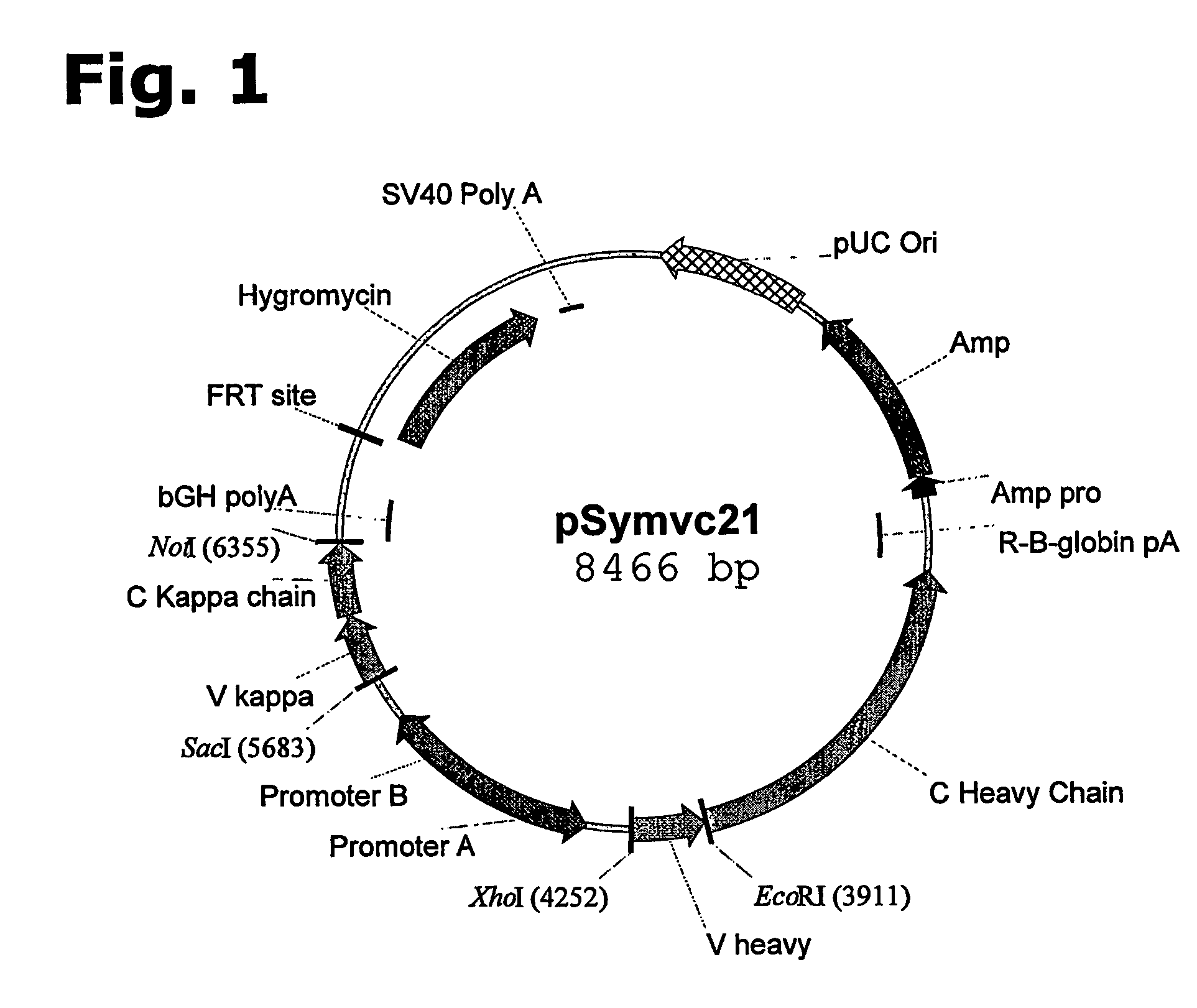
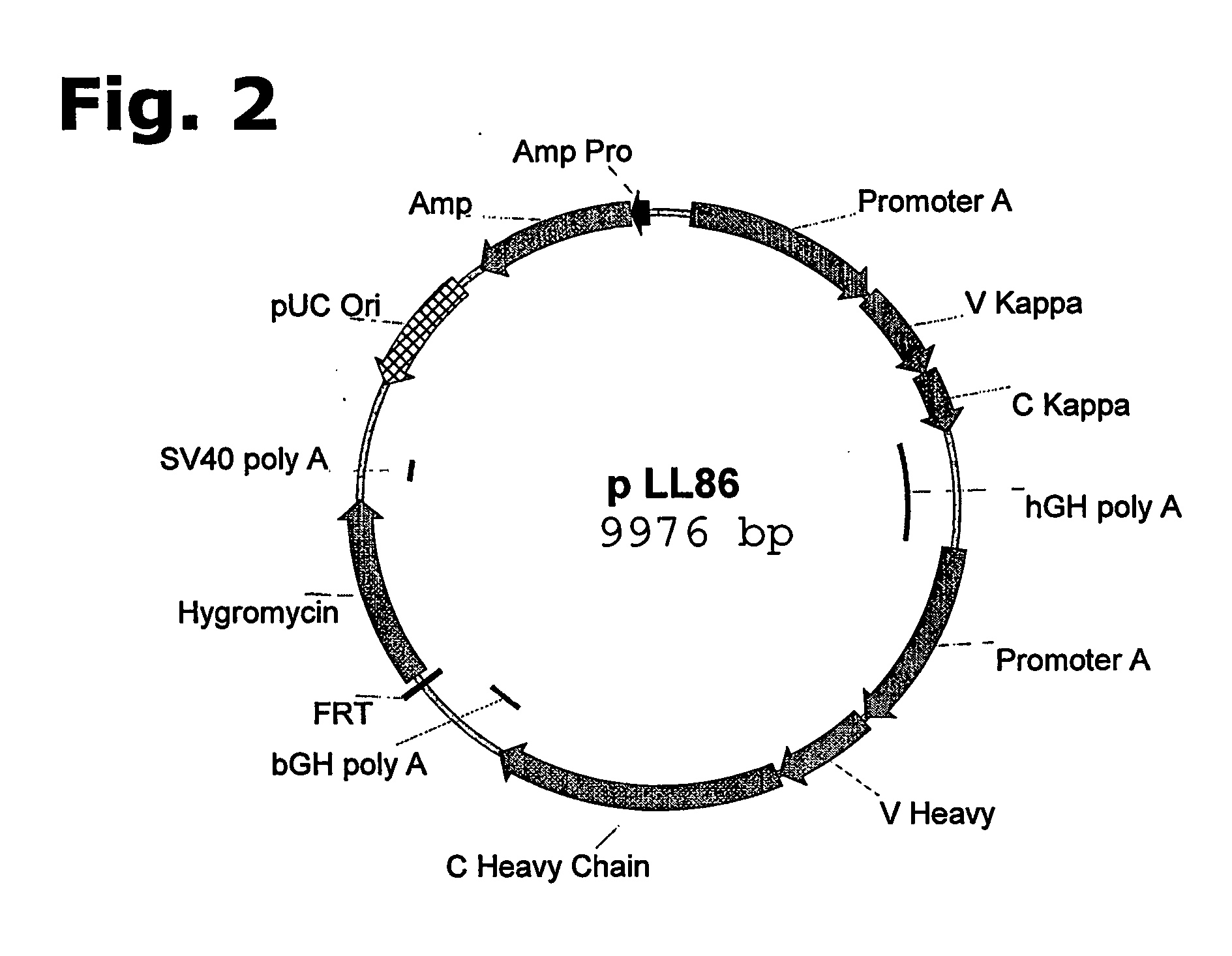
Method for manufacturing recombinant polyclonal proteins
InactiveUS20060275766A1Minimizing unwanted batch-to-batch variationPeptide librariesImmunoglobulins against blood coagulation factorsPresent methodPlasma derived
Owner:SYMPHOGEN AS
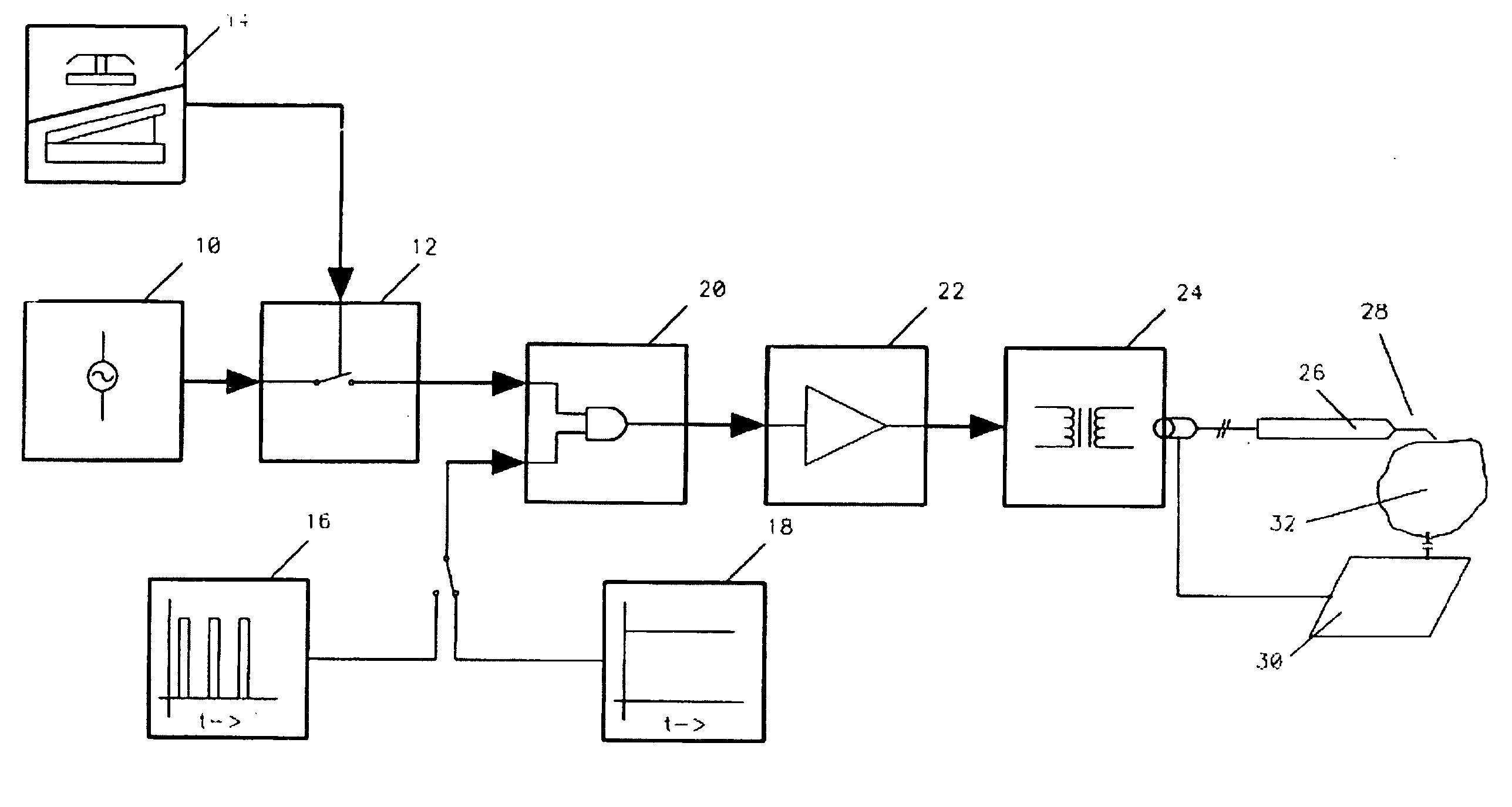
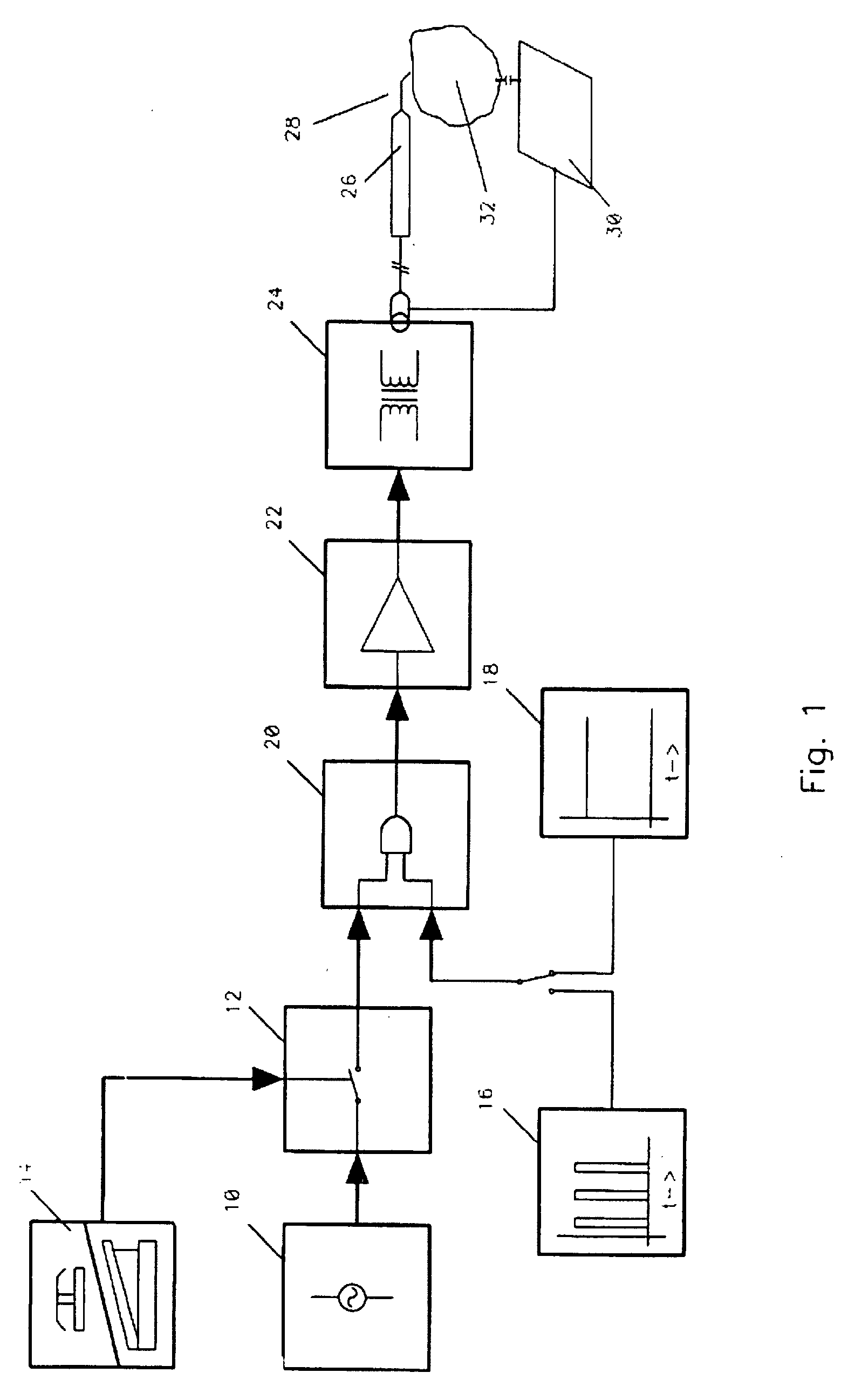
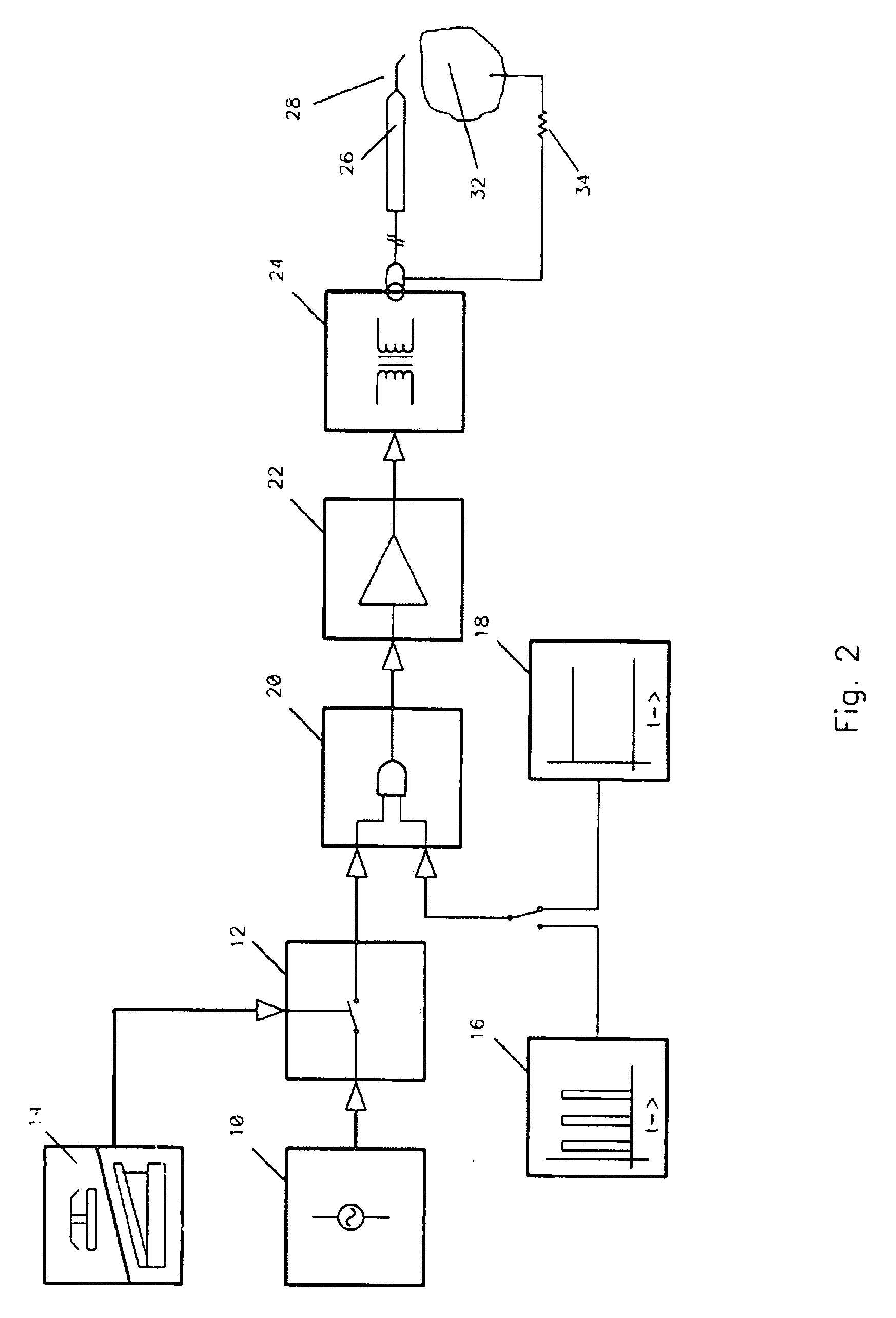
Method and apparatus for plasma incision of cardiovascular tissue
Owner:FUGO RICHARD J
Method for retarding unhealth manifestations brought by ageing of human beings
InactiveUS20090053200A1Reduce functionReduced stress resistancePeptide/protein ingredientsHydrolasesDna antibodyBlood plasma
Owner:CLS THERAPEUTICS
Serum/plasma miRNA composition and use thereof
InactiveCN102021169AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSerum igeProtein markers
Owner:NANJING UNIV
Pulmonary tuberculosis variation activity marker, kit, method and model construction method
PendingCN113178263AGood clinical valueOvercoming low diagnostic detection ratesMedical simulationProteomicsLung tuberculosisBlood plasma
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
Probe composition for detecting lung cancer mutant genes based on NGS method and kit
ActiveCN110791500AStrong specificityHigh and uniform coverageMicrobiological testing/measurementDNA/RNA fragmentationNucleotideBlood plasma
The invention discloses a probe composition for detecting lung cancer gene mutation based on an NGS method and a kit thereof. The probe composition is selected from at least one of probes with nucleotide sequences as shown in SEQ ID NO.1-75, the kit is suitable for lung cancer gene mutation detection of FFPE, tissue and peripheral blood ctDNA based on the NGS method, and then the purposes of earlyscreening of lung cancer mutant genes, real-time monitoring of recurrence and the like are achieved. The uniquely designed UMI bimolecular tag can effectively reduce background noise, eradicate tracepollution, remove false positive and ensure the accuracy of a result, so that the sensitivity in ctDNA detection reaches 0.1%. A universal Short-Y joint is used in tissue detection, and the detectionsensitivity can reach 2%. The more possibilities are provided for accurate targeted therapy of patients. Tissue samples and plasma samples are similar in library building workflow, the simplicity ofthe workflow is guaranteed, time is saved, the efficiency is high, and the operation is easy.
Owner:KEAN BIOTECHNOLOGY (DALIAN) CO LTD
Non-resistant low-zinc low-copper non-plasma high-grade piglet creep feed and preparation method thereof
InactiveCN109845885ABlock homologous diseaseReduce pollutionFood processingAnimal feeding stuffHydrolysateThreonine
Owner:HENAN XUBAIRUI BIOTECH CO LTD
Curcumin compositions and uses thereof
Owner:THE OHIO STATES UNIV
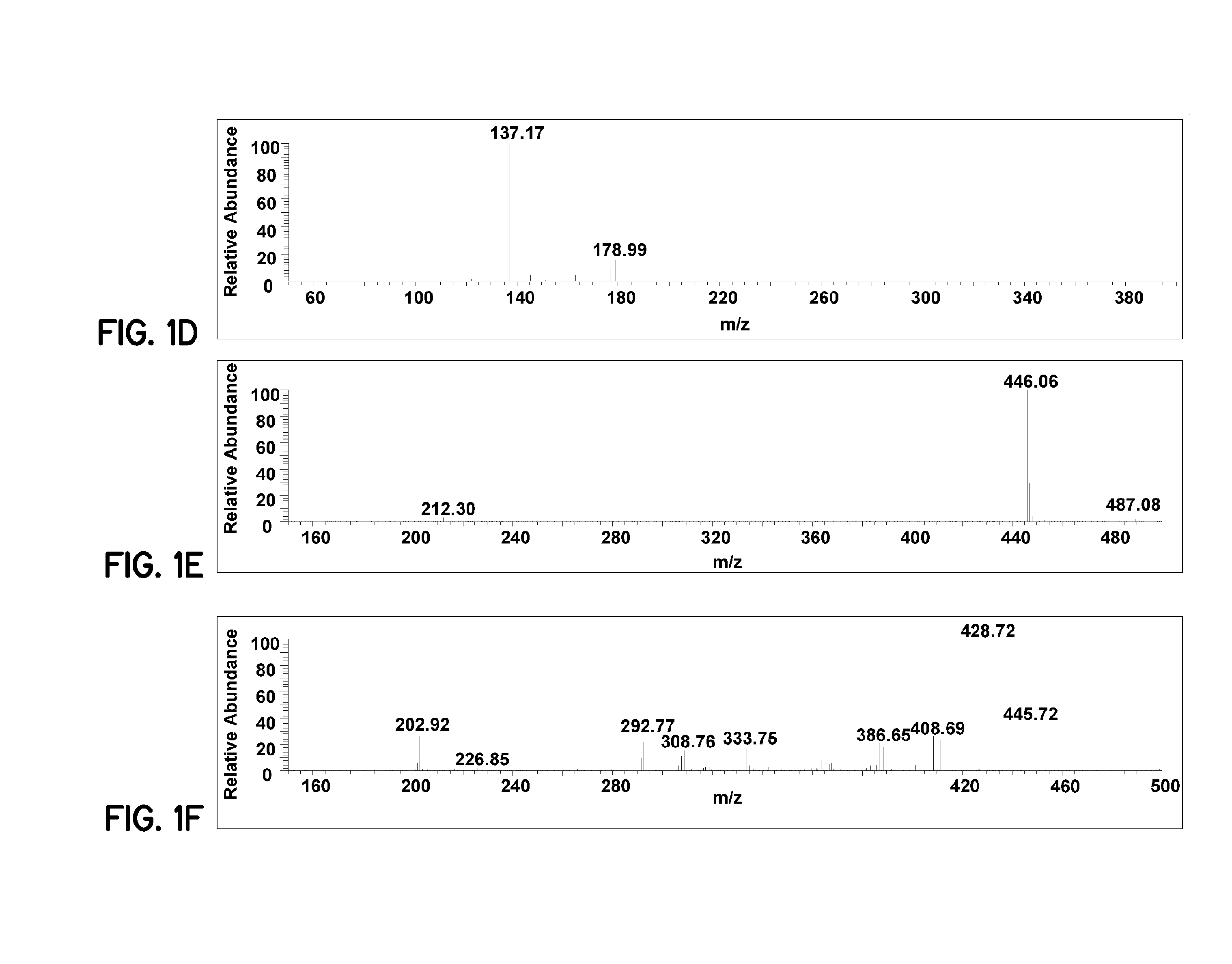
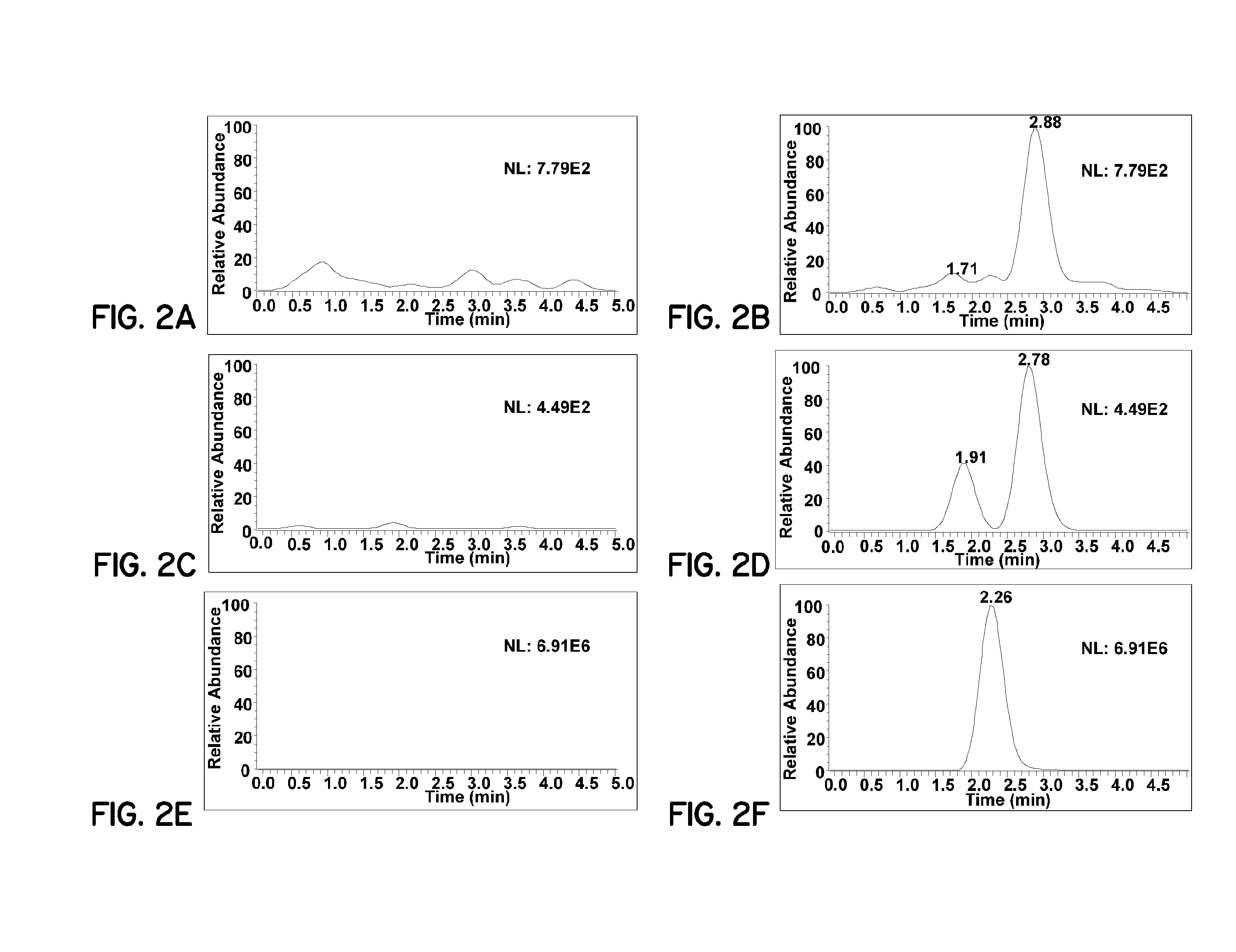
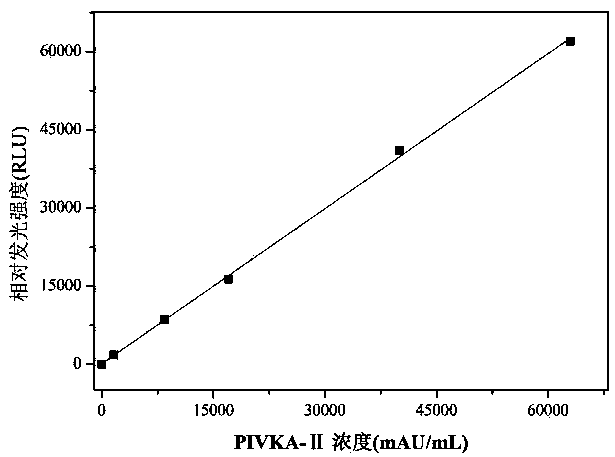
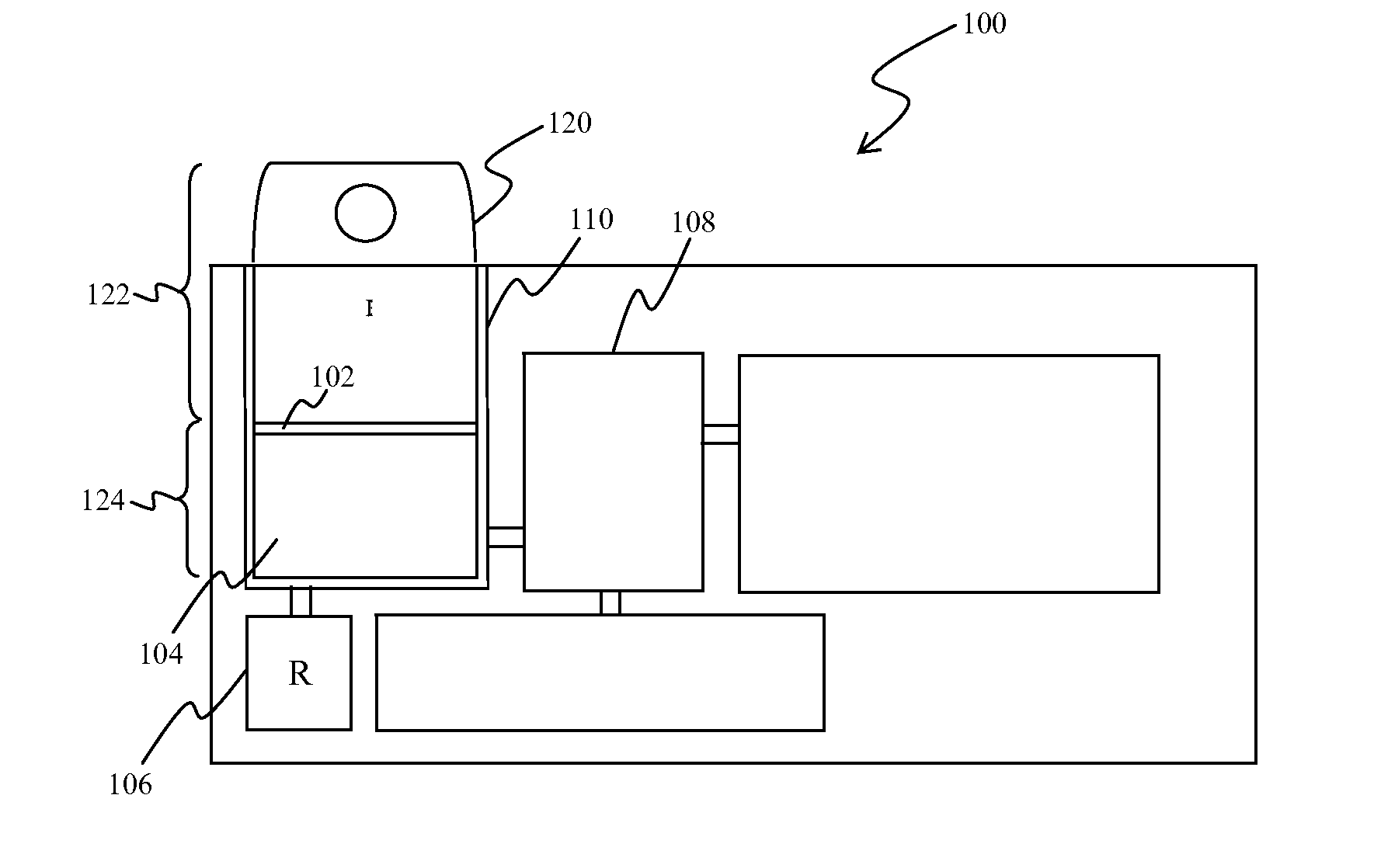
PIVKA-II (protein induced by vitamin K absence-II) magnetic particle chemiluminescence immune assay determination kit and preparation method thereof
PendingCN109425740AHigh sensitivityEasy to operateMaterial analysisBlood plasmaFluorescein isothiocyanate
Owner:江苏麦得科生物科技有限公司
Resurrection factor purification and extraction process for improving injection-type autologous fat survival rate
InactiveCN106754695AEasy extractionKeep activeBlood/immune system cellsProsthesisRemove bloodCentrifugation
Owner:티안지우안
Refining and extracting method of dextran by membrane separation
ActiveCN102988989AHigh purityImprove product qualitySemi-permeable membranesBlood disorderFiltration membraneSeparation technology
Owner:JIANGSU JIUWU HITECH
Whole Blood Analytic Device And Method Therefor
Owner:QUALIGEN INC
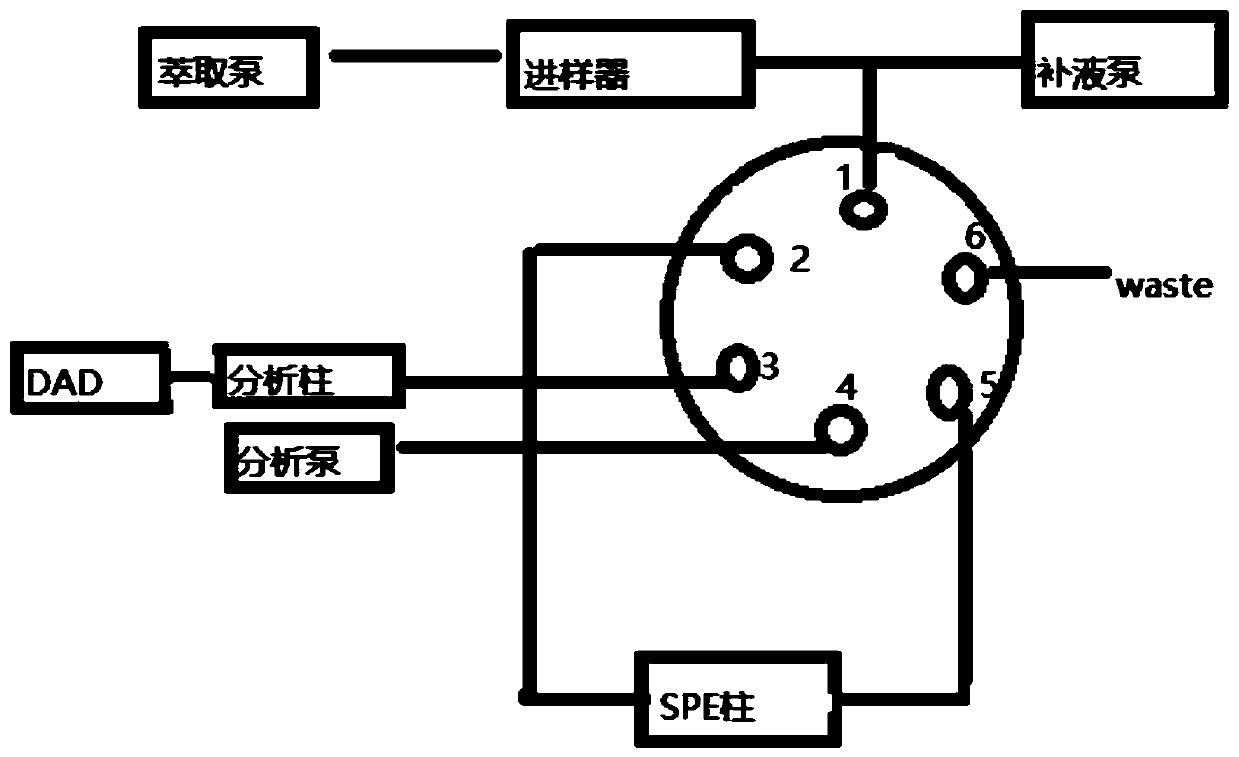
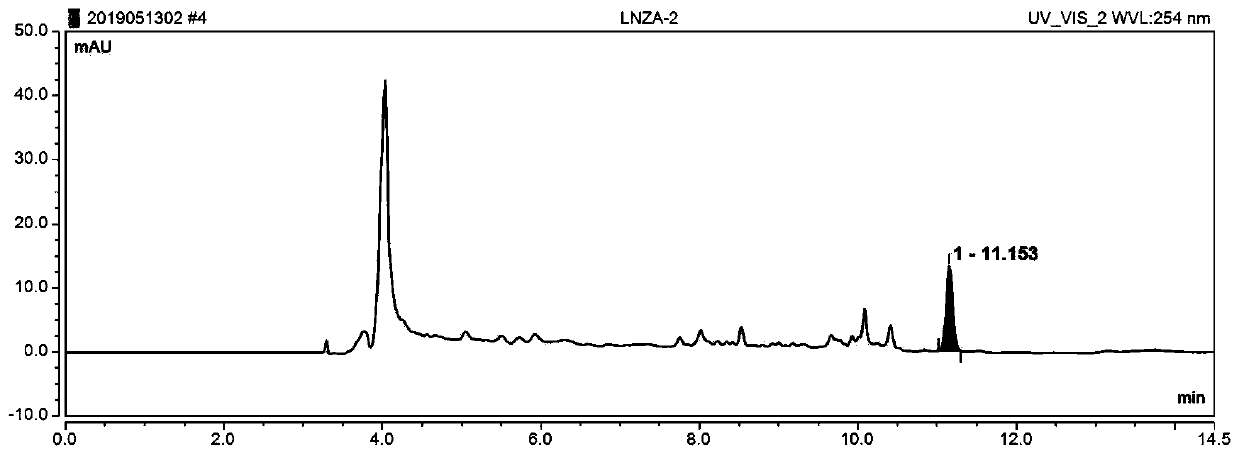
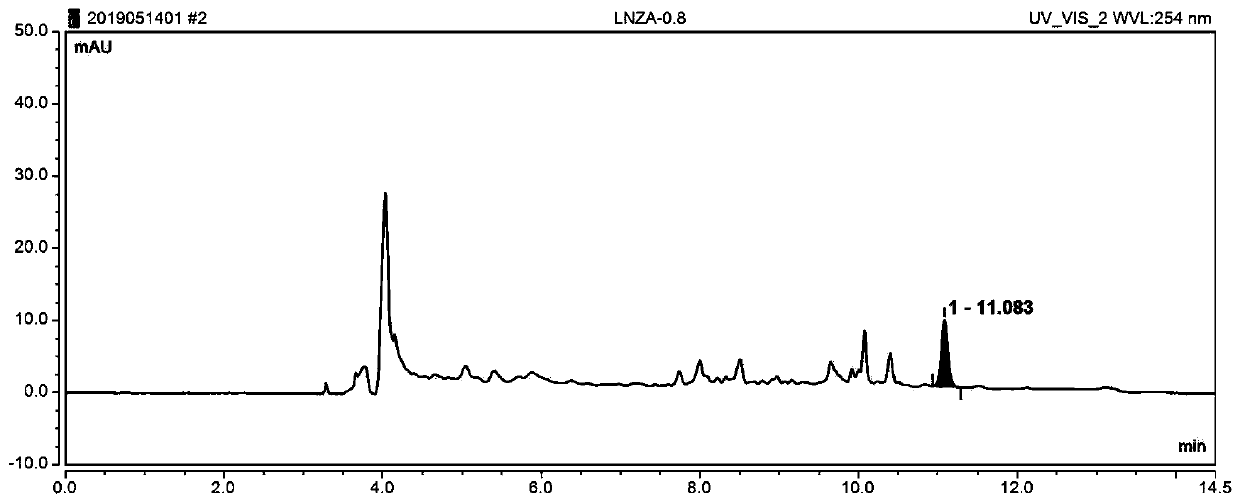
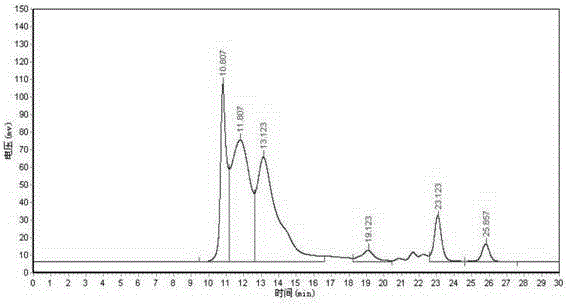
Online solid phase extraction liquid chromatography for detecting content of linezolid in blood
InactiveCN110715998AReduce stepsImprove accuracyComponent separationAntimicrobial drugSolid phase extraction
Owner:JASAN BIO MEDICINE JIAXING CO LTD
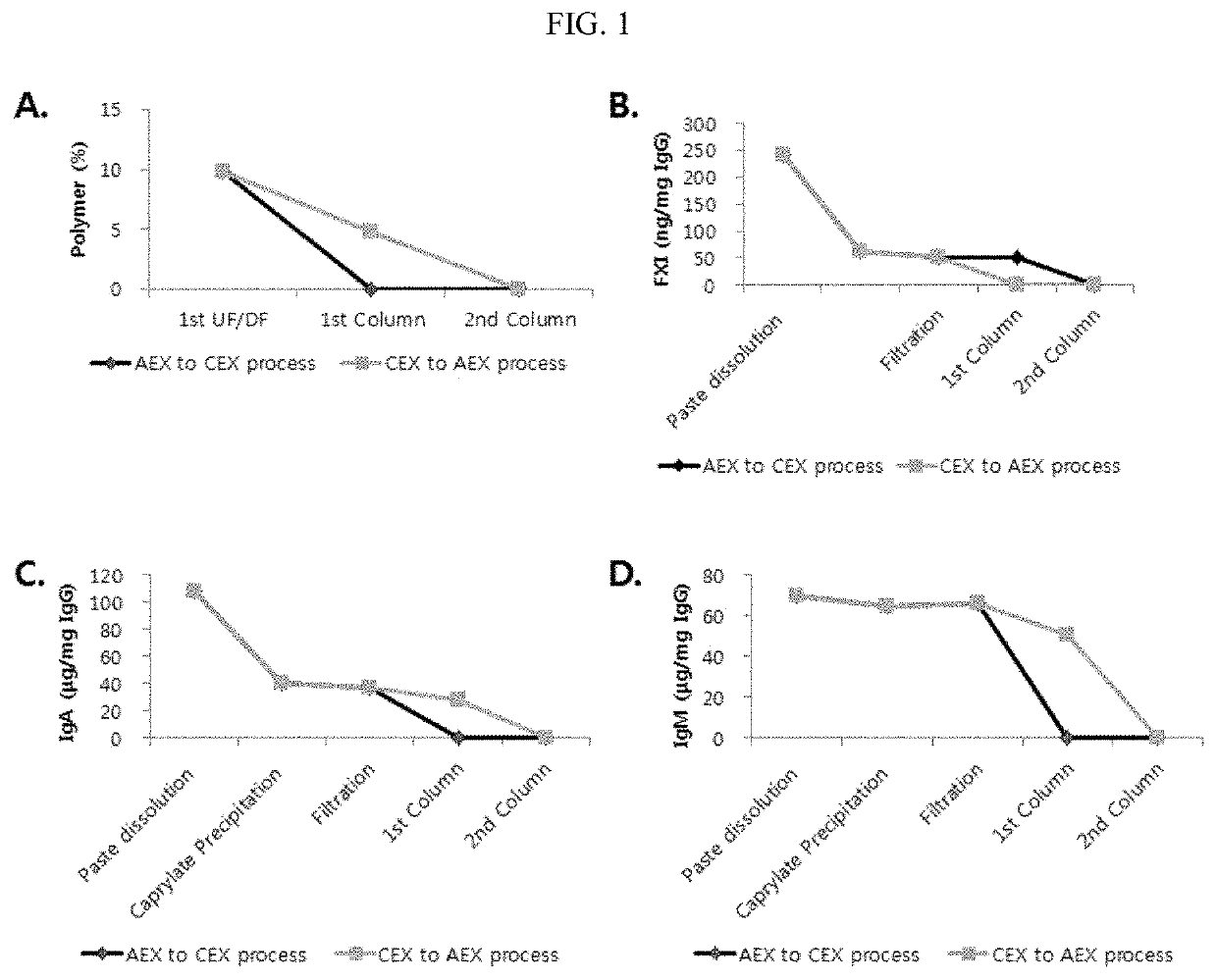
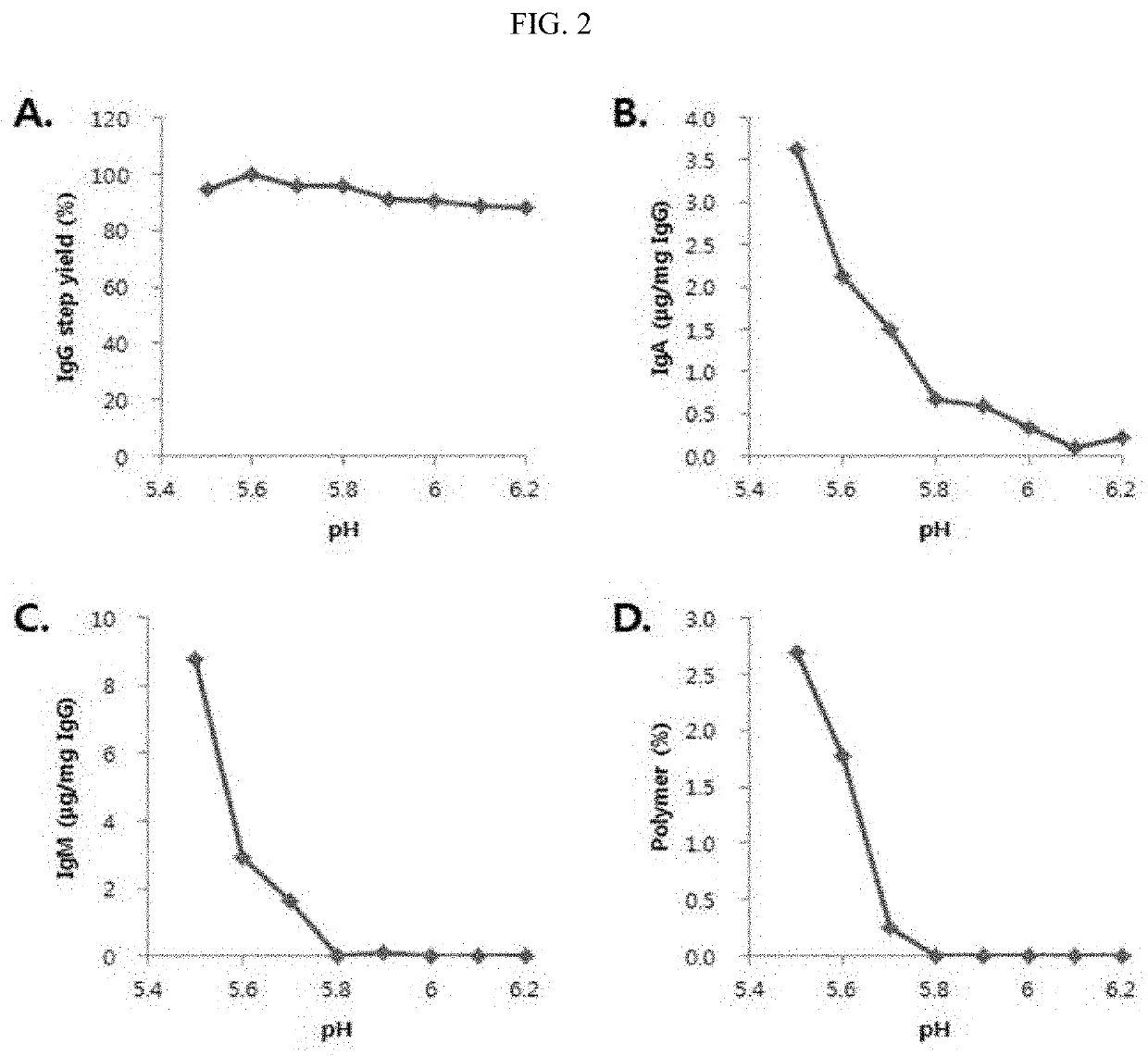
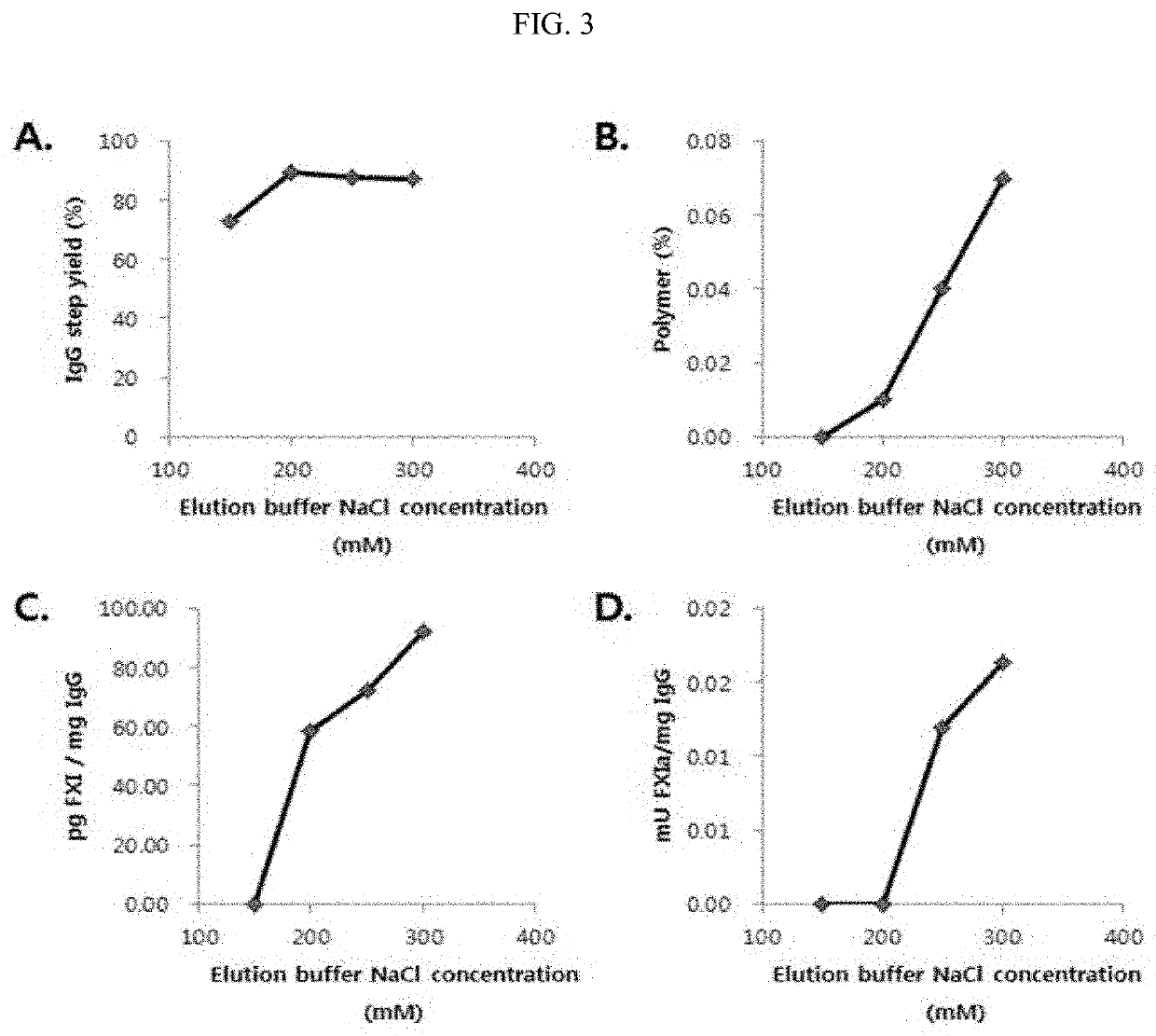
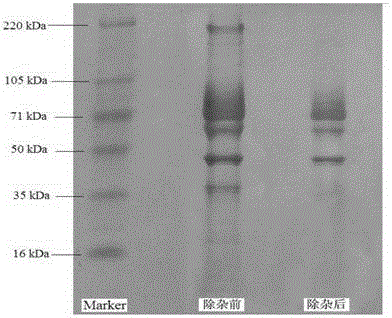
Improved method for purification of immunoglobulin
ActiveUS20200347093A1Serum immunoglobulinsPeptide preparation methodsBlood plasmaIntravenous gammaglobulin
Owner:THE GREEN CROSS CORP
Whole-liver bioartificial liver system
ActiveCN112604051AAdjust the perfusion pressureGood internal and external environmentOther blood circulation devicesWhole liverBlood plasma
The invention provides a whole-liver bioartificial liver system which comprises a plasma separation and circulation passage, a liver perfusion circulation passage, a plasma separation and return circulation passage and a blood storage tank which are relatively independent, and the blood storage tank is used for mixing plasma of a patient and an in-vitro liver oxygen carrier; the plasma separation and circulation passage comprises a blood input pipeline, a first plasma separation pipeline and a blood return pipeline, and the first plasma separation pipeline is connected with the blood storage tank; the liver perfusion circulation path comprises a biochemical purification pipeline provided with a whole liver perfusion assembly, and the biochemical purification pipeline is connected with the blood storage tank; the plasma separation and return circulation passage comprises a second plasma separation circulation pipeline and a plasma return pipeline, the input end of the second plasma separation circulation pipeline is connected with the output end of the blood storage tank, and the plasma return pipeline is connected between the second plasma separation circulation pipeline and the blood return pipeline. By arranging the blood storage tank, the perfusion pressure, flow and flow velocity of the in-vitro liver can be better adjusted, the perfusion efficiency is improved, and the circulation time is shortened.
Owner:GUANGDONG UNISUN BIOTECHNOLOGY CO LTD
Improved medicinal compositions comprising buprenorphine and naltrexone
An analgesic composition, in parenteral unit dosage form or in a unit dosage form suitable for delivery via the dermis or mucosa, comprises buprenorphine and an amount of naltrexone such that the ratio by weight of buprenorphine to naltrexone delivered to or reaching the plasma of a patient is in the range 100:1 to 5000:1. The analgesic action of the buprenorphine is potentiated by the low dose of naltrexone. Also provided are a method of treatment of pain and the use of buprenorphine and naltrexone for the manufacture of a medicament.
Owner:RB PHARMA
Medical equipment for platelet-rich plasma gel (PRG) in-vitro negative pressure cutting treatment
ActiveCN114570266AImprove the effect of repairing and regenerating damaged tissueReduce guide portRotary stirring mixersTransportation and packagingMedical equipmentPlatelets blood
Owner:THE FIRST MEDICAL CENT CHINESE PLA GENERAL HOSPITAL
Immunoadsorption blood purification material and preparation method thereof
PendingCN114272913AImprove adsorption capacityGood blood compatibilityOther chemical processesSolid sorbent liquid separationHemodialysisBlood plasma
Owner:GUANGZHOU KONCEN BIOSCI
MULTIPLEX ASSAY FOR DETERMINING beta-AMYLOID 42/40 RATIO IN HUMAN PLASMA SPECIMENS
The present technology relates to methods for diagnosing, monitoring the progression of, assessing the efficacy of treatment of, or assessing risk for development of a neurodegenerative disorder in a patient. These methods are based on determining the ratio of beta-amyloid 42 ("A beta42") to beta- amyloid 40 ("A beta40") in a body fluid sample collected from a patient who has or is suspected of having a neurodegenerative disorder, using an improved and highly sensitive multiplex protein assay that simultaneously detects A beta42 and A beta40.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
A kind of preparation method of thrombin
Owner:长春雷允上药业有限公司
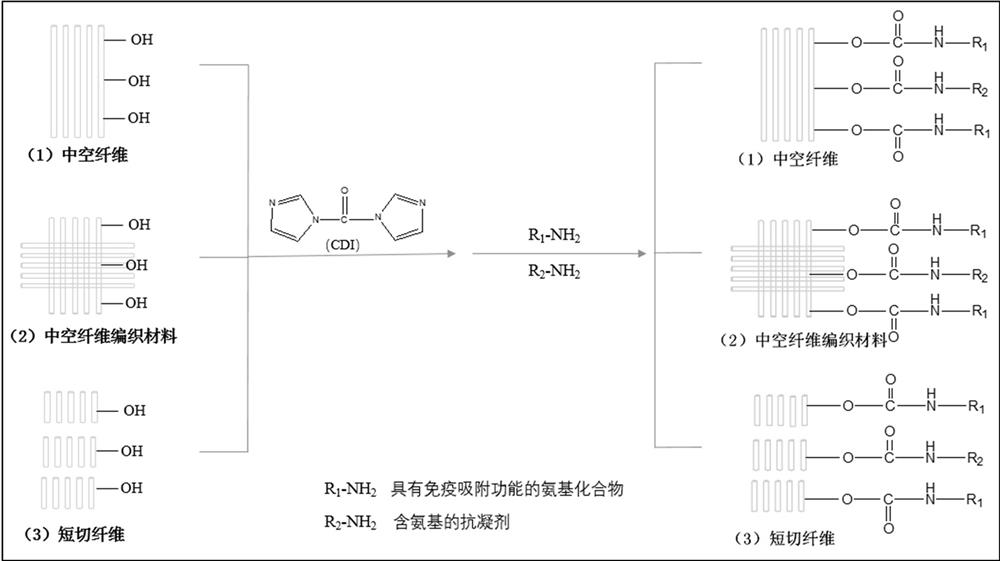
Method of preparing chromatographic materials
InactiveCN105793301AIncrease switching capacityHydrophobic/hydrophilic balanceOther chemical processesSolid sorbent liquid separationCross-linkIntravenous gammaglobulin
The invention relates to a sorbent material. The sorbent material comprises a plurality of cross linked monovinyl monomers defining a matrix, in a ratio of the volume of hydrophobic monovinyl monomers to hydrophilic monovinyl monomers of approximately 5:95 to approximately 40:60, the matrix being bufferable to pH ranges from approximately 5 to approximately 9, and, of particle sizes between approximately 10 micrometers to approximately 300 micrometers. The sorbent is used in chromatographic columns to promote binding of Immunoglobulin G (IgG) from blood plasma, for its isolation, such that the isolated IgG is then extracted from the sorbent. The sorbent is also used in chromatographic columns to promote binding of Immunoglobulin G (IgG) monoclonal antibodies from transgenic milk, for their isolation, such that the isolated IgG monoclonal antibodies are then extracted from the sorbent.
Owner:NEW PROTEINTECH
Dietary supplement formulations for improved delivery of coenzyme Q10 and methods of administration
Owner:JARROW FORMULAS INC
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap